ORIGINAL REPORT
EFFECTS OF DIAGNOSTIC TIBIAL NERVE BLOCK AND SELECTIVE TIBIAL NERVE NEUROTOMY ON SPASTICITY AND SPASTIC CO-CONTRACTIONS: A RETROSPECTIVE OBSERVATIONAL STUDY
Jean-Philippe LAMORA, PT, MSc1,2, Thierry DELTOMBE, MD3 and Thierry GUSTIN, MD4
From the 1UCLouvain Faculty of Motor Sciences, Place Pierre de Coubertin, BE-1348 Louvain-la-Neuve, Belgium, 2La Musse School of Physiotherapy, La Renaissance Sanitaire - Hopital La Musse, CS 20119, 27180 Saint-Sebastien de Morsent, France, 3Physical Medicine and Rehabilitation Department, Université catholique de Louvain, CHU UCL Namur site Godinne, BE-5530 Yvoir, Belgium and 4Neurosurgery Department, Université catholique de Louvain, CHU UCL Namur site Godinne, BE-5530 Yvoir, Belgium
Objective: To assess the effects of diagnostic nerve block and selective tibial neurotomy on spasticity and co-contractions in patients with spastic equinovarus foot.
Methods: Among 317 patients who underwent a tibial neurotomy between 1997 and 2019, 46 patients who met the inclusion criteria were retrospectively screened. Clinical assessment was made before and after diagnostic nerve block and within 6 months after neurotomy. A total of 24 patients underwent a second assessment beyond 6 months after surgery. Muscle strength, spasticity, angle of catch (XV3), passive (XV1) and active (XVA) ankle range of motion were measured. The spasticity angle X (XV1–XV3) and paresis angle Z (XV1–XVA) were calculated with the knee in flexed and extended positions.
Results: Tibialis anterior and triceps surae strength remained unchanged, while both Ashworth and Tardieu scores were highly reduced after nerve block and neurotomy at all measurement times. XV3 and XVA increased significantly after block and neurotomy. XV1 increased slightly after neurotomy. Consequently, spasticity angle X and paresis angle Z decreased after nerve block and neurotomy.
Conclusion: Tibial nerve block and neurotomy improve active ankle dorsiflexion, probably by reducing spastic co-contractions. The results also confirmed a long-lasting decrease in spasticity after neurotomy and the predictive value of nerve blocks.
LAY ABSTRACT
Selective tibial nerve neurotomy is an effective surgical treatment for spastic equinovarus foot deformity after stroke. However, its effectiveness on spastic co-contractions, defined as a disabling involuntary antagonist contraction during an active agonist movement, is unknown. This retrospective study evaluated the effects of tibial neurotomy on the active ankle dorsiflexion limitation related to co-contractions. Selective tibial neurotomy allows an immediate improvement in active dorsal flexion of the ankle, probably by decreasing muscle co-contractions around this joint. This effect continues for the long term for the soleus muscle. This study also confirms that reflex spasticity is permanently reduced after neurotomy. This surgical technique therefore seems useful in limiting impairment caused by the development of spasticity and co-contractions after a stroke.
Key words: spastic co-contractions; active ankle dorsiflexion; tibial neurotomy; spasticity; spastic muscle overactivity; equinovarus foot; denervation; muscle spasticity.
Citation: J Rehabil Med 2023; 55: jrm4850. DOI: https://doi.org/10.2340/jrm.v55.4850
Copyright: © Published by Medical Journals Sweden, on behalf of the Foundation for Rehabilitation Information. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Feb 14, 2023; Published: Jun 12, 2023
Correspondence address: Jean-Philippe Lamora, La Musse School of Physiotherapy, La Renaissance Sanitaire - Hopital La Musse, CS 20119, 27180 Saint-Sebastien de Morsent, France. E-mail: jeanphilippe.lamora.mk@gmail.com
Competing interests and funding: The authors have no conflicts of interest to declare.
Spastic equinovarus foot has an incidence of 18% (in France after a one year follow up cohort study) within the stroke population (1). Spastic equinovarus foot following stroke is responsible for impairment of static and dynamic balance, with gait instability, leading to severe functional limitations (2, 3). Spastic equinovarus foot results from interaction between the tibialis anterior and lateral peronei muscles weakness or imbalance, and overactivity and/or contracture of the calf muscles (triceps surae and tibialis posterior). These negative (paresis) and positive (spastic muscle overactivity) symptoms are characteristic of upper motor neurone syndrome (4). Spastic muscle overactivity refers to several types of muscle hypertonia, including stretch hyperreflexia (spasticity), spastic dystonia, spastic co-contractions, associated reactions (e.g. overflow) and spasms (5). Spasticity is defined as a ‘’velocity dependent increase in tonic stretch reflexes […] with exaggerated tendon jerks” (6). Spastic dystonia is an involuntary tonic muscle hypertonia at rest without any muscle stretch, associated with spasticity (4). Spastic co-contractions are characterized by an involuntary antagonist contraction (i.e. triceps surae) during a voluntary agonist activation (i.e. tibialis anterior), resulting in movement limitation or inversion (4). Co-contractions are one of the main causes of disability even more than paresis (7) and spasticity (8), but co-contractions are difficult to identify at the bedside (5). In addition, soft-tissue contracture caused by intrinsic muscle modifications may occur, limiting both passive and active movements (5).
In clinical routine, 2 main scales are used to assess spasticity: the Modified Ashworth Scale (MAS) and the Modified Tardieu Scale (MTS). In order to better evaluate all forms of spastic muscle overactivity, Gracies et al. also proposed a 5-step clinical assessment (9). This algorithm consists of both passive (steps 1 and 2) and active (steps 3–5) assessments, including measurement of the active range of motion (XVA) and the MTS scores. The MTS provides a spasticity grade and quantitative measures of the passive maximal range of motion at low speed (XV1), which enables detection of muscle contracture, and the passive angle of catch at the fastest speed (XV3). Using these data, 2 other angles are calculated. The difference XV1–XV3 is called the spasticity angle X and reflects the amount of stretch hyperreflexia. The difference XV1–XVA is the paresis angle Z, which represents the amount of paresis and spastic co-contractions. This last angle is of great interest, as it provides information about the ability to perform a selected movement more precisely than a simple active angle.
Treatment of spastic equinovarus foot is multi-modal, including botulinum toxin and selective tibial neurotomy (STN) (10). Botulinum toxin is the first-line treatment, with a high level of evidence in the literature (11).
STN is a neurosurgical intervention, comprising a partial and selective section of the motor nerve fascicles or branches innervating spastic muscles. This section interrupts both afferent (Ia and II) and efferent motor (α and γ) fibres underlying the myotatic reflex. It leads to a permanent suppression of spasticity and to a transient muscle weakening, which resolves after 8–12 months due to collateral reinnervation on the efferent pathway. Its goal is to rebalance tonicity between agonist and antagonist muscles (12). STN has been proven to decrease spasticity in the long term and to increase passive and active ankle dorsiflexion (13, 14). It has also been shown that the effects of STN on spasticity and gait kinematics can be predicted by a diagnostic nerve block (DNB) with anaesthetics (15, 16).
The aim of this study is to investigate the effect of DNB and STN on triceps surae spasticity, assessed by the spasticity angle, and on spastic co-contractions, assessed by the ankle dorsiflexion paresis angle. The study hypothesis is that DNB and STN are able not only to reduce stretch hyperreflexia, but also to reduce spastic co-contractions.
METHODS
Design
This study was retrospectively conducted in patients treated for spastic equinovarus foot in our university hospital (Université catholique de Louvain, CHU UCL Namur site Godinne, BE-5530 Yvoir, Belgium). The study protocol was approved by the hospital ethics committee.
Participants
The study population comprised patients who underwent a STN between August 1997 and April 2019. They were treated by an interdisciplinary group and benefited from a preoperative DNB. They were assessed before and after DNB, in the first 6 months following surgery and, for some of them, beyond 6 months. Inclusion criteria were: age over 18 years, a disabling spastic equinovarus foot improved after DNB (i.e. spasticity and foot deformity reduction with better foot stability), an active ankle dorsiflexion movement of 10° minimum with the knee in flexion after and/or before DNB, and a lack of associated contracture (passive ankle dorsiflexion with the knee flexed ≥ 0° after DNB). Exclusion criteria were: recurrence of a central nervous system lesion during follow-up, injection of botulinum toxin in the calf muscles less than 4 months before surgery and between assessments, absence of active ankle dorsiflexion after DNB, a tendon lengthening or transfer procedure associated with STN (except a toe flexor tenotomy), and no follow-up in the first 6 months after surgery.
Interventions and assessment
All patients underwent a selective DNB with anaesthetics performed by the same physician (TD) in order to identify the spastic muscles implicated in spastic equinovarus foot. The motor branches of the tibial nerve were selected in accordance with the clinical examination. A 1-ml dose of lidocaine 2% was injected through a conduction anaesthesia needle (23-gauge, 100-mm length) connected to an electrical stimulator (Viking Synergy EDX, Natus-Nicolet Medical Inc., Middleton, WI, USA), using anatomical landmarks. Injection was performed once an elective contraction of the target muscle at a low intensity of stimulation (0.01 ms, 5 mA) was found. Clinical assessment was made 20 min later.
STN was performed by the same neurosurgeon (TG) under general anaesthesia at the level of the motor nerve branches targeted during DNB (13, 15). The selection of the nerve branches to be sectioned was made during the pre-operative interdisciplinary consultation. The tibial nerve was dissected through a vertical skin incision below the popliteal fossa. The motor nerve branches of the soleus, gastrocnemius, tibialis posterior and flexor hallucis longus muscles were identified using electrical stimulation (12). Once isolated, the nerve fascicles were partially sectioned over a 5-mm length under microscope. The degree of section (50–80%) was determined according to the spasticity grade. Patients were allowed to walk on the day after surgery without any immobilization or casting.
All clinical assessments were performed by the same physician (TD) on a standard examination table. Each evaluation was carried out as follows: firstly, with the patient sitting, the strength of the dorsiflexor muscles was evaluated manually using the Medical Research Council (MRC) scale. Secondly, the patient lay supine on the table and the affected lower limb was placed in a flexed position with the hip at 45°, the knee at 90° and the ankle at 60° (assuming Tardieu’s 0° is the foot in line with the tibia). The soleus spasticity grade was evaluated using the MAS and MTS. XV1, XV3 and XVA were measured using a goniometer with the centre of the heel and the first metatarsophalangeal joint as landmarks. Evaluation was conducted again with the knee extended to test the soleus-gastrocnemius complex. Primary outcome was the paresis angle Z calculated from XV1 and XVA. Secondary outcomes were the spasticity angle X calculated from XV1 and XV3, MRC score and the spasticity grade (MAS and MTS).
Data processing
Due to the retrospective nature of the study, some secondary outcomes data were missing. The number of measures (N) used were noted beneath the outcome in the corresponding tables. For the MAS, the 1+ grade was transformed into 1,5 to preserve the rankings.
Statistical analysis
Normality for quantitative data was evaluated using a Kolmogorov–Smirnov test. As normality was not acceptable, all data were analysed using a Friedman test. If it was significant, 2-by-2 comparisons were made, using a Bonferroni correction for the multi-analyses. A Kruskal–Wallis test was used at the different measurement times to detect an influence of a toe flexor tenotomy or the type of neurotomy. All tests were 2-tailed and were performed using SPSS 26.0 software (SPSS Inc., Chicago, IL, USA). Results were displayed as median [p25; p75] or mean ± SD (range). Only those p-values considered significant (p < 0.05) were displayed.
RESULTS
Between August 1997 and April 2019, 317 patients underwent a STN. A total of 46 patients met the inclusion criteria and had at least an assessment in the first 6 months after surgery (T1). Among them, 24 had a second assessment between the seventh and the nineteenth month after STN (T2). These 2 samples were independently analysed. One patient had a bilateral surgery leading to 2 measurements in both analyses. Details of patient selection are shown in Fig. 1. Patient characteristics are summarized in Table I. In all analyses, there was no significant difference between DNB and T1 or T2 in all measurements. All significant differences were between baseline and the measurement times.
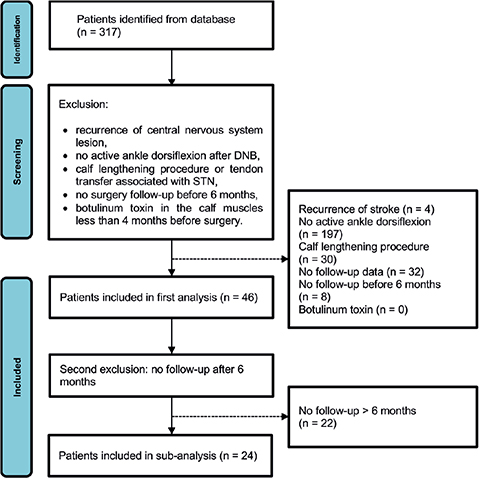
Fig. 1. Data selection flowchart. DNB: diagnostic nerve block; STN: selective tibial neurotomy.
Primary outcome
With the knee in a flexed position, XVA increased after DNB and more significantly after STN at T1 and T2 (Table II). On the other hand, XV1 was only slightly increased at T1. As a result, the paresis angle Z (XV1–XVA) was significantly reduced after STN at both measurement times, while the difference was not significant after DNB.
| Outcome measures | N | Baseline | DNB | T1 < 6 months | T2 > 6 months |
| Knee flexed (soleus) | |||||
| XVA (degrees) | 47 | 90 [80–95] | 90 [90–100]* | 100 [90–100]** | |
| 25 | 90 [80–90] | 90 [90–95] | 100 [90–100]* | 100 [90–100]* | |
| XV1 (degrees) | 47 | 100 [95–100] | 100 [95–100] | 100 [100–102,5]* | |
| 25 | 100 [95–100] | 100 [95–100] | 100 [100–105] | 100 [95–100] | |
| Paresis Z angle (degrees) | 47 | 10 [2.5–12,5] | 5 [0–10] | 0 [0–10]* | |
| 25 | 10 [5–10] | 5 [0–10] | 0 [0–10]* | 0 [0–5]* | |
| Knee extended (soleus-gastrocnemius complex) | |||||
| XVA (degrees) | 47 | 70 [70–80] | 85 [70–90]** | 80 [70–90]* | |
| 25 | 70 [70–80] | 85 [80–90]* | 80 [70–90]* | 80 [70–90] | |
| XV1 (degrees) | 47 | 90 [90–95] | 90 [90–100] | 95 [90–100]* | |
| 25 | 92.5 [90–100] | 95 [90–100] | 95 [90–100] | 92.5 [90–100] | |
| Paresis Z angle (degrees) | 47 | 20 [10–27,50] | 10 [5–20]* | 10 [5–25] | |
| 25 | 20 [15–25] | 10 [5–20]* | 10 [5–20]* | 10 [10–30] | |
| Results are displayed as median [p25; p75]. p-values not displayed are not significant. *p < 0.05 vs baseline. **p < 0.001 vs baseline. | |||||
With the knee in an extended position, XVA also increased significantly after DNB and STN at T1 (Table II), while XV1 slightly increased at T1. Consequently, the paresis angle Z decreased significantly after DNB and STN at T1. However, changes in XVA and Z angle were no longer significant at T2. Notably, at baseline, the Z angle was higher when the knee was in extension. There was no influence of flexor digitorum flexor tenotomy or the type of neurotomy.
Secondary outcomes
Regardless of the knee position, a significant increase in the catch angle XV3 was found after DNB and STN at T1 and T2, with a resulting decrease in the spasticity angle X (XV1–XV3) which became zero in the flexed position (Table III). Spasticity grades (MAS and MTS) also decreased to zero at all measurement times.
| Outcome measures | N | Baseline | DNB | T1 < 6 months | T2 > 6 months |
| MRC | |||||
| Plantar flexion | 44 | 4 [2–4] | 3 [1–4]* | 3 [1–4] | |
| 22 | 4 [3–4] | 4 [1–4] | 3 [1–4] | 4 [3–4] | |
| Dorsiflexion [Knee flexed] | 45 | 4 [4–4] | 4 [4–4] | 4 [4–4] | |
| 24 | 4 [4–4] | 4 [4–4] | 4 [4–4] | 4 [4–4] | |
| Knee flexed (soleus) | |||||
| XV3 (degrees) | 40 | 70 [60–77.5] | 100 [90–100]** | 100 [100–100]** | |
| 22 | 70 [65–80] | 100 [90–100]** | 100 [100–105]** | 100 [90–100]** | |
| Spasticity X angle (degrees) | 40 | 30 [20–32.5] | 0 [0–2.5]** | 0 [0–0]** | |
| 22 | 25 [20–30] | 0 [0–5]** | 0 [0–0]** | 0 [0–0]** | |
| Ashworth score | 40 | 2 [2–3] | 0 [0–0]** | 0 [0–0]** | |
| 22 | 2 [2–3] | 0 [0–0]** | 0 [0–0]** | 0 [0–0]** | |
| Tardieu score | 40 | 3 [3–4] | 0 [0–0]** | 0 [0–0]** | |
| 22 | 3 [3–4] | 0 [0–0]** | 0 [0–0]** | 0 [0–0]** | |
| Knee extended (soleus-gastrocnemius complex) | |||||
| XV3 (degrees) | 40 | 60 [60–70] | 90 [80–90]** | 80 [70–95]** | |
| 22 | 65 [60–70] | 90 [80–90]** | 80 [70–95]** | 80 [70–90]* | |
| Spasticity X angle (degrees) | 40 | 30 [20–35] | 10 [2.5–12.5]** | 17.5 [0–20]** | |
| 22 | 27.5 [20–35] | 10 [0–10]** | 12.5 [0–20]** | 15 [0–20]* | |
| Ashworth score | 45 | 3 [3–3] | 1 [1–2]** | 1 [0–2]** | |
| 24 | 3 [3–3] | 1 [0–1.5]** | 1.5 [0–2]** | 2 [0–2]** | |
| Tardieu score | 41 | 3 [3–3] | 1 [1–2]** | 1 [0–2]** | |
| 23 | 3 [2–3] | 2 [0–2]** | 2 [0–2]** | 2 [0–2]** | |
| Results are displayed as median [p25; p75]. p-values not displayed are not significant. *p < 0.05 vs baseline. **p < 0.001 vs baseline. | |||||
In the sub-group comparisons, X angle with the knee in the extended position was significantly lower at T1 after a soleus and gastrocnemius neurotomy (p < 0.05) than after a soleus neurotomy alone. There was no influence of the flexor digitorum tenotomy.
A slight reduction in plantar flexor muscle strength was detected after DNB (p < 0.05) and early after surgery (NS). At T2, the plantar flexor muscle strength returned to baseline values. No change was found in dorsiflexor strength (Table III).
DISCUSSION
This is the first study specifically investigating the effects of DNB and STN on spasticity assessed by means of the spasticity angle and on spastic co-contractions assessed by means of the paresis angle.
Spasticity
A drastic reduction was observed in MAS and MTS scores after DNB and STN, consistent with the literature data demonstrating a permanent suppression of triceps surae spasticity (13–15, 17–24). This effect was confirmed by the decrease in XV3, a reliable, accurate and objective measure of spasticity contribution (25, 26). In addition, the spasticity angle X has a similar evolution to XV3. This angle provides a precise evaluation of stretch hyperreflexia irrespective of muscle contracture (27) and is a simple way to assess impairment caused by spasticity alone. Spasticity angle X could easily be integrated into the routine clinical examination.
Active ankle dorsiflexion
The literature provides limited and heterogeneous data on the effect of STN on active ankle dorsiflexion. Six studies evaluated the effects of STN on dorsiflexor muscle strength using the MRC scale (13–15, 20, 22, 28) and demonstrated no improvement (28), a short-term improvement (14, 20, 22) or a long-term improvement after STN (13, 15). These discrepancies can be explained by the fact that baseline MRC scores were relatively different between the studies, which included patients without voluntary muscle contraction. Moreover, the MRC scale does not consider the range of movement or the influence of the antagonist muscle overactivity or contracture.
Three studies showed a significant improvement in active range of motion at short- and long-term after STN (14, 17, 22). Bollens et al. (28) also described a significant increase in the maximal ankle dorsiflexion during the stance and the swing phase at 6 months following STN, using instrumented gait analysis. As voluntary activation of dorsiflexor muscles is a prerequisite to demonstrate an improvement in ankle dorsiflexion consecutive to a reduction in triceps co-contractions after STN, the current study included only patients who had an active dorsiflexion movement of a minimum 10° at baseline. In addition, patients presenting a triceps surae contracture were excluded from the study.
After DNB and STN, an increase in active dorsiflexion was observed, which was not correlated with an improvement in dorsiflexor muscle strength but with a reduction in the paresis Z angle. Indeed, immediate changes, the strengthening of dorsiflexors cannot be explained by rehabilitation as it will take much longer to be observed. This suggests that triceps surae spastic co-contractions participate in the limitation of active dorsiflexion and that STN is able to reduce them.
Physiopathology of spastic co-contractions
Clinically, limitation of active ankle dorsiflexion is probably much more related to co-contractions than to an overactive response to phasic stretch of plantar flexors. Physiopathological mechanisms of spastic co-contractions seem more complex. On a spinal level, they could be induced either by a lack of increase in Ia reciprocal inhibition and pre-synaptic inhibition of motor neurones of antagonist muscles at the onset of dorsiflexion (29) or by a decrease in Ib inhibition in the antagonist during an agonist contraction (30). These spinal inhibitory mechanisms are under the control of descending pathways. Another suggested mechanism is a misdirected command at the supraspinal level (31). Recently, a direct link was identified between electroencephalogram (EEG) activity in the β-band and spastic co-contractions in a stroke population (32), providing further evidence for the role of the supraspinal centres. The amount of co-contraction was also found to increase with the degree of antagonist stretching and was higher with the knee extended (33). Similar observations were made in the current study, the Z angle being larger in this knee position.
STN acts mainly on stretch hyperreflexia through interruption of the afferent fibres Ia. Section of afferent fibres Ib and II could also modify inhibitory regulation at the spinal level. Therefore, it could be assumed that STN can reduce the spastic stretch-sensitive component of co-contractions. On the other hand, STN implies a partial section of efferent motor fibres α, leading to a transient triceps surae muscle weakening that recovers after several months through collateral reinnervation. This action on the efferent pathway might also explain the temporary effectiveness of STN on co-contractions by rebalancing the tone of agonist and antagonist muscles. Recently, it was suggested that co-contractions may be related to poor coping strategies to erroneous somato-sensory information with a decrease in the signal-to-noise ratio (34). STN, through attenuation of the sensory signal, could help to reduce the amount of noise induced by spasticity and could therefore limit maladaptive co-contractions around the ankle.
Finally, a more modest effect of STN on the paresis Z angle was observed when the knee was extended. This might be explained by the above hypothesis of stretch-sensitive co-contractions, but also by the study design. All patients benefited from a soleus neurotomy, whereas only a quarter had a gastrocnemius neurotomy. Indeed, the soleus muscle is mainly responsible for clonic spasticity (35, 36) and additional section of the gastrocnemius nerves could weaken foot propulsion during gait. However, the gastrocnemius muscle seems more involved in spastic co-contractions than the soleus (33). Therefore, the effects on active range of motion and Z angle of an extended STN to gastrocnemius nerves could merit further investigation.
Diagnostic nerve block usefulness
As demonstrated in the literature (15, 19, 20), DNB is a valuable screening tool to predict the effects of STN on spasticity, evaluated both by the spasticity angle and by MAS and MTS. However, the predictivity of DNB regarding the effect of surgery on the paresis angle, co-contractions and active function is much lower, especially in the long term.
Study limitations
This study has several limitations, many of which are related to its retrospective nature. Some data were missing in the patients’ files and only a small group of patients were followed up after 6 months. Evolution of techniques over time has also to be considered, e.g. gastrocnemius neurotomy was performed more frequently at the beginning of the study period. The study nonetheless conforms to clinical reality.
Secondly, the co-contraction index was not calculated using electromyography. In this study, the effect of surgery on co-contractions was inferred indirectly from the paresis angle. Without electromyography, it is also difficult to rule out a contribution of spastic dystonia in the reduction of active ankle dorsiflexion. Lack of instrumented gait analysis is another limitation.
Finally, angles were measured with a goniometer, which induces a margin of error. Moreover, X angle reliability varies in the literature: from “good to excellent” inter- and intra-rater after training in children (26) to “acceptable” for the ankle of brain-injured adults (25). Some authors do not recommend spasticity angle (X angle), as it sums measurement errors, but rather suggest using XV3 variations in clinical practice (25). Reliability of the paresis Z angle was never documented.
CONCLUSION
STN enables an immediate improvement in active ankle dorsiflexion and the angle of paresis, independent of the knee position. This effect is probably achieved by reducing co-contractions around this joint. The effect remains in the long term for the soleus muscle, but tends to decline over time for the soleus-gastrocnemius complex.
As expected, reflex spasticity is also permanently reduced after STN, whatever the assessment method used (MAS, MTS or the spasticity angle). In parallel, DNB is a valuable tool to predict the effect of STN on spasticity, but its usefulness in estimating postoperative active ankle dorsiflexion is less clear.
Prospective studies including electromyography and instrumented gait analyses are needed to confirm the action of STN on spastic co-contractions.
ACKNOWLEDGEMENTS
The authors thank Céline Bugli of the “Support en Méthodologie et Calcul Statistique (SMCS)” of UCLouvain for help with statistical analyses and Clare Doyle for reviewing the English in this paper.
REFERENCES
- Verdié C, Daviet JC, Borie MJ, Popielarz S, Munoz M, Salle JY, et al. Epidemiology of varus equinus one year after an hemispheral stroke. Ann Phys Rehabil Med 2004; 47: 81–86. DOI: 10/d8s5ns.
- Giannotti E, Merlo A, Zerbinati P, Prati P, Masiero S, Mazzoli D. Safety and long-term effects on gait of hemiplegic patients in equinovarus foot deformity surgical correction followed by immediate rehabilitation: a prospective observational study. Eur J Phys Rehabil Med 2019; 55: 169–175. DOI: 10/grqwd2.
- Baker JM. Gait disorders. Am J Med 2018; 131: 602–607. DOI: 10/gdn77c.
- Gracies J-M. Pathophysiology of spastic paresis. II: emergence of muscle overactivity. Muscle Nerve 2005; 31: 552–571. DOI: 10/cnkb3g.
- Baude M, Nielsen JB, Gracies J-M. The neurophysiology of deforming spastic paresis: a revised taxonomy. Spec Issue Spasticity 2019; 62: 426–430. DOI: 10/grqwd9.
- Lance JW. Symposium synopsis. In: Feldman RG, Young RR, Koella WP, editors. Spasticity, disordered motor control. Chicago, IL: Year Book Medical Publishers; 1980, p. 485–494. ISBN: 9780883721285.
- Wissel J. Pathophysiology of spasticity and therapeutic approach. In: Sandrini G, Homberg V, Saltuari L, Smania N, Pedrocchi A, editors. Advanced technologies for the rehabilitation of gait and balance disorders. Cham: Springer International Publishing; 2018, p. 449–469. DOI: 10.1007/978-3-319-72736-3_30.
- Chalard A, Amarantini D, Tisseyre J, Marque P, Tallet J, Gasq D. Spastic co-contraction, rather that spasticity, is associated with impaired active function in adults with acquired brain injury: a pilot study. J Rehabil Med 2019; 51: 307–311. DOI: 10/grqwd6.
- Gracies J-M, Bayle N, Vinti M, Alkandari S, Vu P, Loche CM, et al. Five-step clinical assessment in spastic paresis. Eur J Phys Rehabil Med 2010; 46: 411–421.
- Deltombe T, Wautier D, De Cloedt P, Fostier M, Gustin T. Assessment and treatment of spastic equinovarus foot after stroke: guidance from the Mont-Godinne interdisciplinary group. J Rehabil Med 2017; 49: 461–468. DOI: 10/grqwd7.
- Olver J, Esquenazi A, Fung VSC, Singer BJ, Ward AB. Botulinum toxin assessment, intervention and aftercare for lower limb disorders of movement and muscle tone in adults: international consensus statement. Eur J Neurol 2010; 17: 57–73. DOI: 10/cfmf8m.
- Sindou M, Georgoulis G, Mertens P. Peripheral neurotomies. In: Sindou M, Georgoulis G, Mertens P, editors. Neurosurgery for spasticity: a practical guide for treating children and adults. Vienna: Springer; 2014, p. 109–139. DOI: 10.1007/978-3-7091-1771-2_8.
- Deltombe T, Gustin T. Selective tibial neurotomy in the treatment of spastic equinovarus foot in hemiplegic patients: a 2-year longitudinal follow-up of 30 cases. Arch Phys Med Rehabil 2010; 91: 1025–1030. DOI: 10/bd7gt3.
- Rousseaux M, Buisset N, Daveluy W, Kozlowski O, Blond S. Long-term effect of tibial nerve neurotomy in stroke patients with lower limb spasticity. J Neurol Sci 2009; 278: 71–76. DOI: 10/c294zw.
- Deltombe T, Bleyenheuft C, Gustin T. Comparison between tibial nerve block with anaesthetics and neurotomy in hemiplegic adults with spastic equinovarus foot. Ann Phys Rehabil Med 2015; 58: 54–59. DOI: 10/grqwdw.
- Deltombe T, Jamart J, Hanson P, Gustin T. Soleus H reflex and motor unit number estimation after tibial nerve block and neurotomy in patients with spastic equinus foot. Neurophysiol Clin Clin Neurophysiol 2008; 38: 227–233. DOI: 10/b67zzw.
- Feve A, Decq P, Filipetti P, Verroust J, Harf A, N’Guyen JP, et al. Physiological effects of selective tibial neurotomy on lower limb spasticity. J Neurol Neurosurg Psychiatry 1997; 63: 575–578. DOI: 10/cvnb45.
- Bollens B, Deltombe T, Detrembleur C, Gustin T, Lejeune T, Stoquart G. Effects of selective tibial nerve neurotomy as a treatment for adults presenting with spastic equinovarus foot: a systematic review. J Rehabil Med 2011; 43: 277–282. DOI: 10/ftq8kh.
- Buffenoir K, Decq P, Hamel O, Lambertz D, Perot C. Long-term neuromechanical results of selective tibial neurotomy in patients with spastic equinus foot. Acta Neurochir (Wien) 2013; 155: 1731–1743. DOI: 10/f46j3g.
- Le Bocq C, Rousseaux M, Buisset N, Daveluy W, Blond S, Allart E. Effects of tibial nerve neurotomy on posture and gait in stroke patients: a focus on patient-perceived benefits in daily life. J Neurol Sci 2016; 366: 158–163. DOI: 10/f8vwvf.
- Deltombe T, Gilliaux M, Peret F, Leeuwerck M, Wautier D, Hanson P, et al. Effect of the neuro-orthopedic surgery for spastic equinovarus foot after stroke: a prospective longitudinal study based on a goal-centered approach. Eur J Phys Rehabil Med 2018; 54: 853–859. DOI: 10/grqwdz.
- Rousseaux M, Buisset N, Daveluy W, Kozlowski O, Blond S. Comparison of botulinum toxin injection and neurotomy in patients with distal lower limb spasticity. Eur J Neurol 2008; 15: 506–511. DOI: 10/bt6tv6.
- Deltombe T, Detrembleur C, Hanson P, Gustin T. Selective tibial neurotomy in the treatment of spastic equinovarus foot: a 2-year follow-up of three cases. Am J Phys Med Rehabil 2006; 85: 82–88. DOI: 10/fmjdv6.
- Sindou M, Mertens P. Selective neurotomy of the tibial nerve for treatment of the spastic foot. Neurosurgery 1988; 23: 738–744. DOI: 10/cxcz9t.
- Ben-Shabat E, Palit M, Fini NA, Brooks CT, Winter A, Holland AE. Intra- and interrater reliability of the modified Tardieu scale for the assessment of lower limb spasticity in adults with neurologic injuries. Arch Phys Med Rehabil 2013; 94: 2494–2501. DOI: 10/f5jq33.
- Gracies J-M, Burke K, Clegg NJ, Browne R, Rushing C, Fehlings D, et al. Reliability of the Tardieu scale for assessing spasticity in children with cerebral palsy. Arch Phys Med Rehabil 2010; 91: 421–428. DOI: 10/c8x327.
- Gracies J-M. Coefficients of impairment in deforming spastic paresis. Ann Phys Rehabil Med 2015; 58: 173–178. DOI: 10/gkqfq4.
- Bollens B, Gustin T, Stoquart G, Detrembleur C, Lejeune T, Deltombe T. A randomized controlled trial of selective neurotomy versus botulinum toxin for spastic equinovarus foot after stroke. Neurorehabil Neural Repair 2013; 27: 695–703. DOI: 10/f5hp7n.
- Morita H, Crone C, Christenhuis D, Petersen NT, Nielsen JB. Modulation of presynaptic inhibition and disynaptic reciprocal Ia inhibition during voluntary movement in spasticity. Brain J Neurol 2001; 124: 826–837. DOI: 10/fpjkbk.
- Morita H, Shindo M, Momoi H, Yanagawa S, Ikeda S, Yanagisawa N. Lack of modulation of Ib inhibition during antagonist contraction in spasticity. Neurology 2006; 67: 52–56. DOI: 10/dd4v68.
- Gracies J-M, Wilson L, Gandevia S, Burke D. Stretched position of spastic muscles aggravates their co-contraction in hemiplegic patients. Ann Neurol 1997: 438–439.
- Chalard A, Amarantini D, Tisseyre J, Marque P, Gasq D. Spastic co-contraction is directly associated with altered cortical beta oscillations after stroke. Clin Neurophysiol 2020; 131: 1345–1353. DOI: 10/grq5qv.
- Vinti M, Couillandre A, Hausselle J, Bayle N, Primerano A, Merlo A, et al. Influence of effort intensity and gastrocnemius stretch on co-contraction and torque production in the healthy and paretic ankle. Clin Neurophysiol 2013; 124: 528–535. DOI: 10/f4nct2.
- Nielsen JB, Christensen MS, Farmer SF, Lorentzen J. Spastic movement disorder: should we forget hyperexcitable stretch reflexes and start talking about inappropriate prediction of sensory consequences of movement? Exp Brain Res 2020; 238: 1627–1636. DOI: 10/grq5qt.
- Decq P, Cuny E, Filipetti P, Kéravel Y. Role of soleus muscle in spastic equinus foot. Lancet Lond Engl 1998; 352: 118. DOI: 10/djbzcn.
- Buffenoir K, Rigoard P, Lefaucheur J-P, Filipetti P, Decq P. Lidocaine hyperselective motor blocks of the triceps surae nerves: role of the soleus vs gastrocnemius on triceps spasticity and predictive value of the soleus motor block on the result of selective tibial neurotomy. Am J Phys Med Rehabil 2008; 87: 292–304. DOI: 10/bpp6rs.