ORIGINAL ARTICLE
EFFECTS OF TRANSCRANIAL DIRECT CURRENT STIMULATION FOLLOWED BY TREADMILL TRAINING ON DUAL-TASK WALKING AND CORTICAL ACTIVITY IN CHRONIC STROKE: A DOUBLE-BLINDED RANDOMIZED CONTROLLED TRIAL
Pei-Ling WONG, BS, PT1, Yea-Ru YANG, PhD, PT1, Shih-Fong HUANG, MD2 and Ray-Yau WANG, PhD, PT1
From the 1Department of Physical Therapy and Assistive Technology, National Yang Ming Chiao Tung University and 2Division of Nerve Repair- Department of Neurosurgery, Taipei Veterans General Hospital, Taipei, Taiwan, ROC
Objective: To explore the effects of transcranial direct current stimulation followed by treadmill training on dual-task gait performance and contralesional cortical activity in chronic stroke patients.
Methods: Forty-five chronic stroke participants were randomized into 3 groups: a bilateral transcranial direct current stimulation and treadmill training group; a cathodal transcranial direct current stimulation and treadmill training group; and a sham transcranial direct current stimulation and treadmill training group for 50 min per session (20 min transcranial direct current stimulation followed by 30 min treadmill training), 3 sessions per week for 4 weeks. Outcome measures included cognitive dual-task walking, motor dual-task walking, walking performance, contralesional cortical activity, and lower-extremity motor control.
Results: The cathodal transcranial direct current stimulation + treadmill training group showed significantly greater improvements in cognitive dual-task walking speed than the other groups (pcathodal vs sham = 0.006, pcathodal vs bilateral = 0.016). In the cathodal transcranial direct current stimulation + treadmill training group the silent period duration increased significantly more than in the other groups (p < 0.05). Changes in motor evoked potentials in the cathodal transcranial direct current stimulation + treadmill training group were greater than those in the sham transcranial direct current stimulation + treadmill training group (p < 0.05). No significant changes were observed in the bilateral transcranial direct current stimulation + treadmill training group.
Conclusion: Cathodal transcranial direct current stimulation followed by treadmill training is an effective intervention for improving cognitive dual-task walking and modulating contralesional cortical activity in chronic stroke. No beneficial effects were observed after bilateral transcranial direct current stimulation and treadmill training.
LAY ABSTRACT
Dual-task walking is essential for daily functioning, both at home and socially. This study explored the effects of transcranial direct current stimulation followed by treadmill training on dual-task gait performance and contralesional cortical activity in chronic stroke patients. A total of 45 chronic stroke patients were randomized to 1 of 3 groups: a bilateral transcranial direct current stimulation and treadmill training group, a cathodal transcranial direct current stimulation and treadmill training group, or a sham transcranial direct current stimulation and treadmill training group for 50 min per session, 3 sessions per week for 4 weeks. Cognitive dual-task walking, motor dual-task walking, walking performance, contralesional cortical activity, and lower-extremity motor control of the affected side were measured before and after the intervention. The results show that cathodal transcranial direct current stimulation followed by treadmill training is an effective intervention for improving cognitive dual-task walking and modulating contralesional cortical activity in individuals with chronic stroke.
Key words: transcranial direct current stimulation; treadmill training; dual-task walking; contralesional cortical activity; rehabilitation; chronic stroke.
Citation: J Rehabil Med 2023; 55: jrm00379. DOI: https://doi.org/10.2340/jrm.v55.5258
Copyright: © Published by Medical Journals Sweden, on behalf of the Foundation for Rehabilitation Information. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Jan 31, 2023; Published: Mar 21, 2023
Correspondence address: Ray-Yau Wang, Department of Physical Therapy and Assistive Technology, National Yang Ming Chiao Tung University, 155, Sec. 2, Li-Nong St., Beitou District, Taipei, 112, Taiwan, ROC. E-mail: rywang@nycu.edu.tw
Competing interests and funding: The authors have no conflicts of interest to declare.
Walking ability is impaired in most stroke patients. Gait impairments may include decreased speed, cadence, and step length, and increased stride time (1). Performing another task during walking, dual-task walking (DTW), further negatively impacts walking ability. However, DTW is essential for daily functioning, both at home and socially. Therefore, improving DTW performance is important for stroke rehabilitation. According to the principle of task-specific training, DTW training has been proposed to improve DTW performance (2). However, Liu et al. found that such training programmes did not show better effects on DTW performance than conventional physical therapy (2). Treadmill training (TT) is used as an effective treatment for improving walking performance (3), and has also been reported to improve DTW in stroke patients (4). However, the improvement in DTW speed did not reach the minimal clinically important difference (MCID) in people with stroke (5). Thus, the effectiveness of TT on DTW in patients with stroke remains unclear.
The results of a functional near infrared spectroscopy study demonstrated that brain activity in the premotor cortex and supplementary motor area, especially contralesionally, correlated negatively with speed, cadence, and stride length and positively with stride time during DTW in people with chronic stroke (6). Such findings indicate that contralesional motor-related brain areas are activated more in stroke patients with worse DTW performance. Thus, modulating the contralesional motor area coupled with TT may be a potential training strategy for improving DTW performance in stroke patients.
Transcranial direct current stimulation (tDCS) may alter neural excitability/inhibition by modulating the gamma-aminobutyric acid (GABA) and glutamate systems in the motor cortex (7). A recent meta-analysis showed that cathodal tDCS resulted in positive effects on upper-extremity motor functions due to the suppression of contralesional M1 excitability in chronic stroke (8). In contrast, bilateral tDCS is thought to inhibit contralesional primary motor cortex (M1) and excite ipsilesional M1 to rebalance cortical activities (9). Several studies have reported that applying cathodal tDCS and bilateral tDCS with physical training resulted in greater improvements in hand function in stroke patients (10–12). However, little information is available regarding the effects of tDCS and training on DTW performance. Our recent study demonstrated that 1 session of bilateral or cathodal tDCS improved DTW performance, possibly through inhibiting contralesional M1 in chronic stroke (13). However, the accumulative effects of such combination of tDCS and physical training are not known. Therefore, the objective of this study was to investigate the accumulated effects of combining tDCS (cathodal and bilateral) and TT on DTW performance and contralesional brain activity in individuals with chronic stroke. It was hypothesized that tDCS and TT would result in greater improvements in DTW performance and contralesional brain changes than TT alone in individuals with chronic stroke.
METHODS
Subjects
The study protocol was approved by the ethics committees of Taipei Veterans General Hospital and National Yang-Ming University. This trial was registered at the Thai Clinical Trials Registry (CTR20200910006 on 10 September 2020) and conformed to the Consolidated Standards of Reporting Trials (CONSORT) checklist. Data on stroke diagnosis, age, sex, stroke type, lesion site, and post-stroke duration were obtained from detailed clinical interviews and medical charts. The inclusion criteria were: (i) 6 months after first-ever stroke with unilateral motor deficits; (ii) ability to walk independently a distance of at least 10 m without using walking aids; and (iii) a score of ≥ 24 on the Mini-Mental State Examination (MMSE). Exclusion criteria were: any contraindication to tDCS or transcranial magnetic stimulation (TMS) (e.g. skin lesions on sites for stimulation, metal implants, pacemakers); neurological or orthopaedic diseases that would affect participation in the experiment; past or current history of non-superficial tumours or malignant tumours; or epilepsy. All participants provided signed informed consent before participation.
Experimental design
This study was a double-blinded (assessor- and participant-blinded) randomized controlled trial (RCT) with pre- and post-measurements. After the baseline evaluation, an individual who was not involved in the study selected a sealed envelope to assign participants to 1 of the 3 groups: bilateral tDCS and TT group; cathodal tDCS and TT group; and sham tDCS and TT group. Participants were blinded to the group assignments. Participants in the bilateral tDCS + TT and cathodal tDCS + TT groups received 20 min of bilateral tDCS and cathodal tDCS, respectively, followed by 30 min of TT. Participants in the sham tDCS + TT group received sham tDCS stimulation, followed by 30 min of TT. The 50-min training programme was administered at 3 sessions per week for 4 weeks for a total of 12 sessions by the same physical therapist. The outcomes were measured before the intervention (pre-test) and on the day after completing the 12 training sessions (post-test) by the same assessor, who was blinded to the group assignment (Fig. 1).
Fig. 1. Flowchart of participant inclusion and study procedures (n = 45). tDCS: transcranial direct current stimulation; TT: treadmill training.
Intervention
Transcranial direct current stimulation. The stimulation was delivered by a current stimulator (Eldith DC Stimulator, NeuroConn,Ilmenau,Germany) through a pair of 35 cm2 electrodes with a maximal output of 2 mA. The stimulation intensity was set at 2 mA with a current density of 0.07 C/cm2, which is well below the threshold for tissue damage (14). Electrode placements for the different groups are described as follows: (i) bilateral tDCS: the anode was placed over the ipsilesional M1 and the cathode over the contralesional M1with the current delivered for 20 min (13); (ii) cathodal tDCS: the cathode was placed over the contralesional M1 and the anode over the contralateral supraorbital ridge with the current delivered for 20 min (13); (iii) sham tDCS: the electrodes were positioned as described for cathodal tDCS for 20 min. However, the current was only delivered for 60 s, with a ramp-up and ramp-down of 30 s to mimic the sensation of real tDCS.
Treadmill training. Following tDCS (real or sham), the participants immediately received 30 min of TT (Biodex, Shirley, New York, NY, USA). During training, an experienced physical therapist encouraged the participants to walk with a long stride, symmetry, and upright posture as much as possible. The treadmill speed, which started according to each individual’s comfortable walking speed on level ground, was increased by an increment of 0.2 km/h every 5 min, as tolerated. The criteria for increasing speed were determined by the ability to maintain an upright posture and perceived exertion of “somewhat hard” or lower (i.e. a Borg rating of perceived exertion < 13) (15).
Outcome measures
The primary outcome was DTW performance, and secondary outcomes included walking performance, contralesional cortical activity, and motor control of the affected lower extremity.
Dual-task walking performance. Cognitive DTW (CDTW) and motor DTW (MDTW) performance were measured using the GAITRite system (CIR System Inc., Havertown, PA, USA). CDTW involved walking at a comfortable speed while verbally serial subtracting by 3, starting from a randomized 3-digit number (e.g. 211). MDTW involved walking at a comfortable speed while carrying a tray with a bottle of water with the non-affected hand. There were 3 trials for measuring CDTW and MDTW performance, and the sequence of the 6 trials was random. The mean of the 3 CDTW and MDTW trials was used for data analysis. The GAITRite system is a straight walkway containing pressure-sensitive sensors. The walkway and pressure-sensitive area is 4.75 m long and 0.9 m wide and 4.30 m long and 0.61 m wide, respectively. Concurrent validity has been established, and test-retest reliability while executing dual tasks in stroke subjects has been proven (16, 17). The gait parameters of interest were speed, cadence, step time, and step length. In addition, the dual-task cost of gait speed (DTC-speed) was calculated to indicate dual task interference. DTC-speed = (DTW speed–walking speed)/walking speed ×100% (18).
Walking performance. Walking performance was also measured using the GAITRite system, as described above. Participants walked at a comfortable pace without a secondary task 3 times. The gait parameters of interest were speed, cadence, step time, and step length.
Contralesional cortical activity. As aforementioned, brain activity in the contralesional motor-related areas correlated negatively with gait parameters during DTW in people with chronic stroke (6). Thus, this study measured changes in contralesional cortical activity after the interventions to elucidate the possible mechanisms of the training programmes. The resting motor threshold (RMT) and motor evoked potentials (MEP) were elicited by transcranial magnetic stimulation (TMS) to indicate the cortical excitability of the unaffected M1. In addition, the silent period (SP) duration and short-interval intracortical inhibition (SICI) were measured using TMS to indicate contralesional cortical inhibition.
The MEPs of the unaffected tibialis anterior (TA) muscle were recorded using a Nicolet Viking EDX EMG system (Natus) in response to TMS (Magstim 200 magnetic stimulator; Magstim Co., Whiteland, Dyfed, UK). TMS was delivered through a double-cone coil placed on the contralesional M1, with the participants lying supine comfortably wearing a fitted cap marked with a coordinate system (distance 1 cm). The optimal scalp location (hot spot) was determined by moving the TMS stimulator over the scalp in 1-cm steps. Once the hot spot was identified, a single-pulse TMS was delivered to the location to determine the RMT, defined as the lowest stimulus intensity necessary to elicit MEPs greater than 0.05-mV peak-to-peak amplitude in at least 5 of the 10 consecutive stimuli (19). RMT was expressed as a percentage of the maximum stimulator output. The SP duration was determined during isometric voluntary contraction of the TA muscle. Ten magnetic stimuli were applied at an intensity of 120% RMT while the participant performed a maximum of 20% voluntary contraction. The SP duration was determined from MEP onset to the recurrence of at least 50% of EMG background activity (20). The paired-pulse paradigm was performed in the relaxed TA muscle to assess the SICI. The conditioning stimulus intensity was set at 80% RMT with a short inter-stimulus interval (2 ms), and the testing stimulus at 120% RMT. SICI was expressed as the percentage of inhibition using the following formula:100 – (conditioned MEP/unconditioned MEP) × 100 (21). For each condition, the data of 10 trials were collected and averaged.
Lower extremity motor control. Motor control of the affected lower limb was assessed using the Fugl-Meyer assessment-lower extremity (FMA-LE), which has been reported to have good reliability for people with stroke (22). Each item is scored using a 3-point ordinal scale from 0 (no performance) to 2 (complete performance), with a maximum of 34 points. Higher scores indicated better control of the lower extremities.
Sample size
The sample size was calculated using G*power v3.1.9.7 (Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany). According to a study exploring the effect of gait training combined with tDCS to improve gait performance (effect size f = 0.536) in participants with chronic stroke (23), the effect size f was set to 0.5, with a power of 0.80 and a 2-tailed alpha level of 0.05, in the current study. Assuming a dropout rate of 30%, 45 subjects (15 in each group) were required to detect a significant difference in gait speed.
Statistical analyses
All analyses were performed using SPSS 25.0 (IBM Corp., Armonk, NY, USA). Descriptive statistics (mean (standard deviation; SD), number, or median (interquartile range; IQR)) were calculated for all variables. The Shapiro–Wilk test was used to assess normal distributions. The inter-group differences in baseline characteristics were analysed using 1-way analysis of variance (ANOVA) for continuous variables or χ2 test for nominal scales. Intention-to-treat analysis was used for missing data in the post-test. The normal distribution of outcomes, including gait performance, cortical excitability, and inhibitory and lower limb motor performance, could not be confirmed by the Shapiro–Wilk test, and thus intergroup differences in baseline were evaluated using Kruskal–Wallis 1-way analysis of variance by ranks. The generalized estimating equation (GEE) model was used for repeated measures to analyse the effect of time, group, and time × group interaction. The GEE model was used to evaluate the differential changes in each outcome across the time points between the 3 groups. Differential changes in each outcome were assessed using the regression coefficient (B) of the group × time interaction terms in the model. In addition, pairwise comparisons of the GEE analyses with post hoc Bonferroni correction were performed to estimate the adjusted p-value for within-group changes. The effect sizes for the differences in changing values between groups were calculated using Cohen’s d formula (24). A Cohen’s d value of 0.2 indicates a small effect size, 0.5 indicates, moderate effect size, and 0.8 indicates a large effect size (25). Statistical significance was set at p < 0.05.
RESULTS
A total of 68 stroke participants were screened for eligibility and 45 participants were included from Taipei Veterans General Hospital and randomized to the bilateral tDCS + TT (n = 15),cathodal tDCS + TT (n = 15),or sham tDCS + TT groups (n = 15). None of the patients reported adverse events during the study period. No significant group differences were found in baseline demographic characteristics (Table I) or outcome measures at the pre-intervention assessment.
Cognitive dual-task performance
GEE statistical analysis showed a significant time-group interaction on CDTW speed (Wald χ2 (2) = 8.541, p = 0.014), cadence (Wald χ2 (2) = 10.039, p = 0.007), and step time of the affected side (Wald χ2 (2) = 8.953, p = 0.011) (Table II). The parameter estimates of the model showed that the cathodal tDCS + TT group showed a significantly greater increase in CDTW speed (β = 8.60, p = 0.006) (Fig. 2A), cadence (β = 13.56, p = 0.002), and a greater reduction in step time of the affected side (β = –0.32, p = 0.005) compared with the sham tDCS + TT group. Moreover, the cathodal tDCS + TT group showed a significantly greater increase in CDTW speed (β = 6.77, p = 0.016) (Fig. 2A) and cadence (β = 8.89, p = 0.026) and a greater reduction in step time of the affected side (β = –0.28, p = 0.043) than the bilateral tDCS + TT group. However, there were no significant differences between the bilateral tDCS + TT and sham tDCS + TT groups. Pairwise comparisons from the GEE revealed that only the cathodal tDCS + TT group showed significant improvements in CDTW speed (p < 0.001) and cadence (p = 0.031) (Table SI). The mean values for all the CDT gait parameters are shown in Table SI.
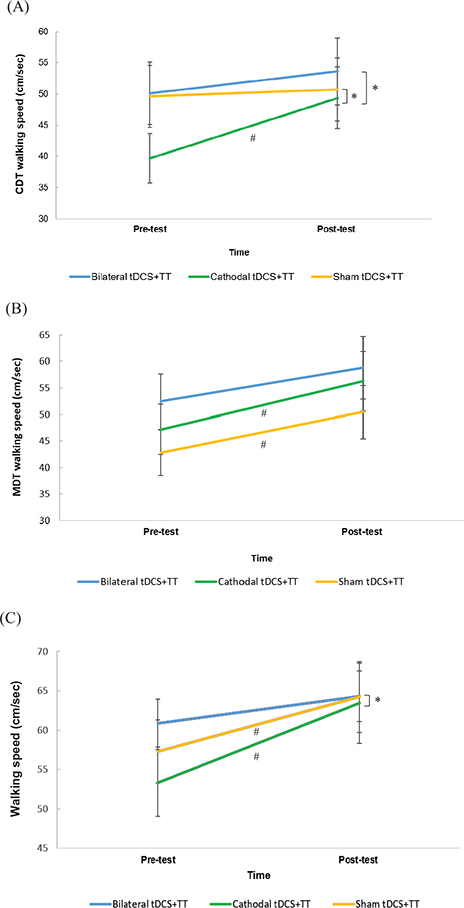
Fig. 2. Effect of different transcranial direct current stimulation (tDCS) followed by treadmill training (TT) on walking speed during: (A) cognitive dual-task walking (CDTW); (B) motor dual-task walking (MDTW); (C) walking performance. Data are presented as the median (interquartile range). #p < 0.05: intra-group comparison; *p < 0.05: time*group interaction.
Motor dual-task performance
No significant time-group interactions on MDTW performance (Table II). Pairwise comparisons of the GEE found that the cathodal tDCS + TT group showed significant improvements in MDTW speed (p = 0.001), cadence (p = 0.007), step length (affected leg, p < 0.001; unaffected leg, p = 0.026), and step time (affected leg, p = 0.036; unaffected leg, p = 0.020) (Table SI). In the sham tDCS + TT group, a significant increase in MDTW speed (p < 0.001), cadence (p = 0.002), step length (affected leg: p < 0.001; unaffected leg: p = 0.014), and step time of the unaffected leg (p = 0.006) was observed after 12 training sessions (Table SI). The mean values for all gait parameters are shown in Table SI.
Walking performance
GEE statistical analysis revealed a significant time-group interaction in the walking speed (Wald χ2 (2) = 7.978, p = 0.019), cadence (Wald χ2 (2) = 6.850, p = 0.033), and step time of the unaffected side (Wald χ2 (2) = 10.617, p = 0.005) (Table III). The interaction effect indicated that the cathodal tDCS + TT group showed significantly greater improvement in cadence (β = 5.82, p = 0.026) and a greater reduction in step time on the unaffected side (β = –0.09, p = 0.003) than the sham tDCS + TT group. The cathodal tDCS + TT group also showed a significantly greater increase in speed (β = 6.70, p = 0.005) (Fig. 2C) and cadence (β = 6.41, p = 0.017) than the bilateral tDCS + TT group. However, there were no significant differences between the bilateral tDCS + TT and sham tDCS + TT groups. Pairwise comparisons revealed that both the cathodal tDCS + TT group (p < 0.001) and sham tDCS + TT group (p = 0.024) showed significant improvements in walking speed after 12 training sessions (Table SII). In contrast, a significant increase in cadence (p = 0.003), step length of the affected side (p < 0.001), and reduction in step time of the unaffected side (p = 0.004) were observed after cathodal tDCS + TT. The mean values for all gait parameters are shown in Table SII.
Contralesional cortical activity
There were significant time-group interactions in MEP (Wald χ2 (2) = 8.171, p < 0.001) and SP (Wald χ2 (2) = 17.498, p < 0.001) (Table III). The cathodal tDCS + TT group had a significantly greater reduction in MEP (β = –162.44, p = 0.004) and a greater increase in SP (β = 7.75, p < 0.001) than the sham tDCS + TT group (Fig. 3). Compared with the bilateral tDCS + TT group, the cathodal tDCS + TT group showed a significantly greater increase in SP (β = 4.76, p = 0.003). There were no significant differences between the bilateral tDCS + TT and sham tDCS + TT groups. The cathodal tDCS + TT group had significant increases in RMT (p = 0.012) and SP (p < 0.001), and a reduction in MEP (p < 0.001) of the contralesional hemisphere (Table SIII). Furthermore, a significant increase in SP (p = 0.013) was observed in the sham tDCS + TT group. No significant changes were found in the bilateral tDCS + TT group.
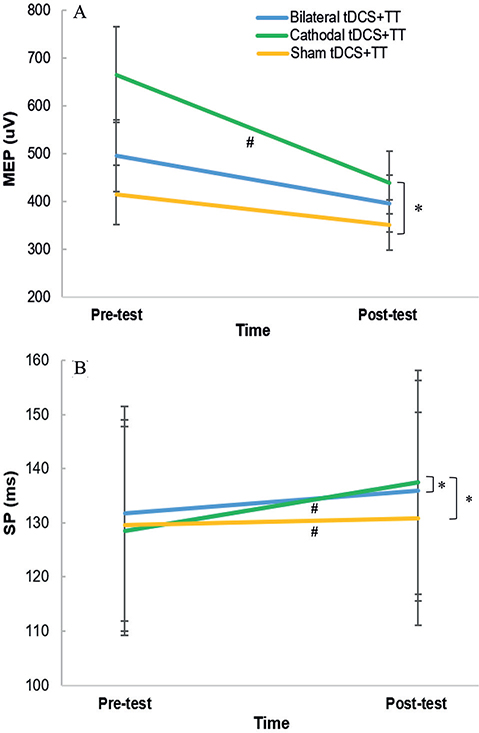
Fig. 3. Changes in cortical activity of contralesional hemisphere after different transcranial direct current stimulation (tDCS) followed by treadmill training (TT). (A) motor-evoked potentials (MEP); (B) silent period (SP). Data are presented as median (interquartile range). #p < 0.05: intra-group comparison; *p < 0.05: time*group interaction.
Lower extremity motor control
The GEE demonstrated a significant time-group interaction in lower extremity motor control (Wald χ2 (2) = 8.314, p = 0.016) (Table III). Compared with the sham tDCS + TT group, the FMA-LE scores improved more in the cathodal tDCS + TT group (β = 0.57, p = 0.006). The pairwise comparisons revealed that only the cathodal tDCS + TT group had a significant increase in the FMA-LE scores (p = 0.002) (Table SIII).
DISCUSSION
This randomized controlled trial is the first study to explore the effects of inhibiting the contralesional motor area followed by TT on DT gait performance and contralesional cortical activity in individuals with chronic stroke. Both cathodal tDCS and bilateral tDCS were applied for inhibitory modulation of the contralesional motor area in the current study. It was found that cathodal tDCS followed by TT can result in better effects on the CDTW speed, cadence, and step time of the affected leg than TT alone. Regarding cortical activity, cathodal tDCS and TT significantly increased inhibition and decreased the excitability of the contralesional M1 more than TT alone. However, 12 sessions of bilateral tDCS and TT showed no significant effects on DTW performance or cortical activity changes in participants with chronic stroke.
In line with the current results, Nair et al. demonstrated that cathodal tDCS combined with occupational therapy improved upper-extremity motor function in chronic stroke patients (26). However, Fusco et al. did not show beneficial effects of cathodal tDCS followed by physical training on gait performance in participants with acute stroke (27). The authors speculated that the lack of a cathodal tDCS effect may be due to time after stroke. The results of a meta-analysis also indicated that the positive effects of cathodal tDCS on motor performance were found only in chronic stroke, but not in acute or subacute stroke (8). As the contralesional M1 has been considered to support motor recovery in early stroke, it plays an interfering role in chronic stroke (28, 29). Therefore, the current findings support the hypothesis that cathodal tDCS followed by TT exerts positive effects on CDTW in chronic stroke. It was further noted that the increased gait speed during CDTW reached the MCID level (23%) (5). Collett et al. reported that 20 sessions of treadmill walking with or without cognitive distraction significantly improved CDTW in the good walkers (walking speed ≥ 0.8 m/s), but not in the limited walkers (walking speed < 0.79 m/s) after stroke (30). However, up to 79% of people with stroke are categorized as limited walkers (31). Of note, in the current study, 35 participants (77.8%) could be categorized as limited walkers, 10 participants in the bilateral tDCS + TT group (walking speed = 36.78 (17.0) cm/s), 14 participants in the cathodal tDCS + TT group (walking speed = 36.06 (11.2) cm/s), and 11 participants in the sham tDCS + TT group (walking speed = 34.36 (16.0) cm/s). After subgroup analysis, it was noted that the CDTW speed of limited walkers in the cathodal tDCS + TT group improved more than that in the sham tDCS + TT group (p = 0.038). This highlights that cathodal tDCS and TT may be effective interventions to improve CDTW ability in individuals with chronic stroke, including limited walkers. Also, the findings of the current study suggest the potential transferal of improvements in CDTW in daily activities, such as talking or answering a phone when walking.
Parallel with improvement in CDTW, cortical excitability and inhibition demonstrated by MEP and SP, respectively, also changed significantly after cathodal tDCS and TT. Furthermore, changes in CDTW speed correlated positively with changes in SP of the contralesional hemisphere (Spearman’s correlation ρ = 0.585, p = 0.028), but not MEP after cathodal tDCS and TT. Previous studies reported that participants demonstrating worse CDTW performance showed increased brain activation in contralesional motor areas during CDTW (2, 30). Corp et al. found that better DTW performance may be related to longer SP in older adults (32). Taken together, we suggest that the increase in inhibitory modulation in the contralesional M1 after 12 sessions of cathodal tDCS and TT may be related to improvements in CDTW performance in people with chronic stroke. However, the change in SICI was not significant after cathodal tDCS or TT. Therefore, in people with chronic stroke, the SICI measured at rest may be insufficient to reveal motor impairments, as the SP measured during isometric contraction (33). The results of a meta-analysis indicated that the difference between the ipsilesional and contralesional hemispheres in chronic stroke was only shown by the SP, but not by the SICI (34). Thus, the SICI may not be sensitive enough to document intracortical inhibition and indicate motor recovery in chronic stroke. In contrast, the neurophysiological phenomenon of SP is thought to be mediated through the GABAB-ergic system (35), while SICI is suggested to be at least partly GABAA receptor-mediated (36). According to the current results, the GABAB-ergic system may play a role in the mechanisms of cathodal tDCS in brain activity modulation after stroke.
However, cathodal tDCS and TT did not exert a better effect on MDTW performance than TT alone. Both cathodal tDCS combined with TT and TT alone improved MDTW performance significantly, including speed, cadence, step length, and step time (Table SI). Thus, TT alone may improve MDTW performance; however, it is necessary to validate such findings in RCT studies, since the current study only reported improvements after TT. This emphasized that the improvements in MDTW speed did not correlate with the changes in SP and MEP after 12 sessions of cathodal tDCS and TT. In line with the current findings, a previous study also demonstrated that only CDTW performance was related to changes in SP, but not MDTW performance in older adults (32). Thus, it is reasonable to speculate that the neuromechanisms underlying the improvement in CDTW and MDTW may vary. Furthermore, we suggest that CDTW can be incorporated in future studies, not only because of its functional importance, but also its measurement sensitivity.
Similar to MDTW performance, walking performance was improved by both cathodal tDCS followed by TT and TT alone, and adding cathodal tDCS did not exert a better effect than TT alone. Fusco et al. also reported that walking speed improved after cathodal tDCS combined with exercise, and after exercise only, and that there was no difference in the improvement between these 2 groups (27). Therefore, we suggest that applying cathodal tDCS before TT for 12 sessions may be particularly effective in improving CDTW ability, but not MDTW or walking ability, compared with TT in people with chronic stroke.
Surprisingly, bilateral tDCS and TT did not exert positive effects on CDTW, MDTW, or walking performance. Similarly, previous studies have reported that bilateral tDCS combined with general exercise did not significantly improve gait performance compared with general exercise in those with chronic stroke (37, 38). According to Naro et al.’s study (39), the motor cortex is prone to interhemispheric imbalance if bilateral tDCS is applied prior to robot-aided gait training. They also demonstrated that bilateral tDCS applied concurrently with gait training or after gait training exerted better gait improvements than those applied prior to gait training in people with chronic stroke (39). It is not clear whether the bilateral tDCS and TT protocol in the current study resulted in an interhemispheric imbalance, since the study only measured the motor cortex in the contralesional hemisphere. However, no significant effects on walking performance were noted after 12 sessions of bilateral tDCS followed by TT.
After 12 training sessions, it was found that cathodal tDCS and TT improved FMA-LE scores more than TT alone. A previous study showed that 10 sessions of 1 Hz rTMS followed by task-oriented training significantly improved FMA-LE scores in patients with chronic stroke (40). In this study, we further noted that the changes in FMA-LE correlated with changes in MEP of the contralesional M1 after cathodal tDCS and TT (Spearman’s correlation ρ = 0.612, p = 0.034). Similarly, Sehle et al. reported that upper extremity training effects on FMA scores were positively related to MEP amplitudes of the ipsilesional M1 in stroke patients (41). Therefore, modulation of cortical excitability after cathodal tDCS and TT may be related to improvements in FMA-LE after chronic stroke.
Study limitations
This study has some limitations. First, the study demonstrated the effects of tDCS followed by TT, and the effects of tDCS prior to, or concurrent with, TT are unknown. Secondly, the cortical activities were assessed only in the contralesional hemisphere, and the changes in the lesional hemisphere were not provided after tDCS, which limited the understanding of bilateral hemispheric interaction after intervention. Thirdly, the study did not measure follow-up changes; therefore, it could not demonstrate whether the observed positive effects were maintained.
CONCLUSION
The positive effects of cathodal tDCS followed by TT on CDTW performance and contralesional cortical activity were superior to those of TT alone in patients with chronic stroke. However, bilateral tDCS and TT did not significantly affect DTW performance and cortical activity.
ACKNOWLEDGEMENTS
This work was supported by grants from the Ministry of Science and Technology (MOST-106-2314-B-010-037-MY3) and National Health Research Institutes (NHRI-EX110-10913PI).
REFERENCES
- Balaban B, Tok F. Gait disturbances in patients with stroke. PM R 2014; 6: 635–642. DOI: 10.1016/j.pmrj.2013.12.017.
- Liu YC, Yang YR, Tsai YA, Wang RY. Cognitive and motor dual task gait training improve dual task gait performance after stroke – a randomized controlled pilot trial. Sci Rep 2017; 7: 4070. DOI: 10.1038/s41598-017-04165-y.
- Patterson SL, Rodgers MM, Macko RF, Forrester LW. Effect of treadmill exercise training on spatial and temporal gait parameters in subjects with chronic stroke: a preliminary report. J Rehab Res Dev 2008; 45: 221–228. DOI: 10.1682/jrrd.2007.02.0024.
- Meester D, Al-Yahya E, Dennis A, Collett J, Wade DT, Ovington M, et al. A randomized controlled trial of a walking training with simultaneous cognitive demand (dual-task) in chronic stroke. Eur J Neurol 2019; 26: 435–441. DOI: 10.1111/ene.13833.
- Parati M, Ambrosini E, DE Maria B, Gallotta M, Dalla Vecchia LA, Ferriero G, et al. The reliability of gait parameters captured via instrumented walkways: a systematic review and meta-analysis. Eur J Phys Rehabil Med 2022; 58: 363–377. DOI: 10.23736/S1973-9087.22.07037-X.
- Liu YC, Yang YR, Tsai YA, Wang RY, Lu CF. Brain activation and gait alteration during cognitive and motor dual task walking in stroke – a functional near-infrared spectroscopy study. IEEE Trans Neural Syst Rehabil Eng 2018; 26: 2416–2423. DOI: 10.1109/TNSRE.2018.2878045.
- Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist 2011; 17: 37–53. DOI: 10.1177/1073858410386614.
- Kang N, Weingart A, Cauraugh JH. Transcranial direct current stimulation and suppression of contralesional primary motor cortex post-stroke: a systematic review and meta-analysis. Brain Inj 2018; 32: 1063–1070. DOI: 10.1080/02699052.2018.1481526.
- Lindenberg R, Renga V, Zhu LL, Nair D, Schlaug G. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology 2010; 75: 2176–2184. DOI: 10.1212/WNL.0b013e318202013a.
- Zimerman M, Heise KF, Hoppe J, Cohen LG, Gerloff C, Hummel FC. Modulation of training by single-session transcranial direct current stimulation to the intact motor cortex enhances motor skill acquisition of the paretic hand. Stroke 2012; 43: 2185–2191. DOI: 10.1161/STROKEAHA.111.645382.
- Lefebvre S, Laloux P, Peeters A, Desfontaines P, Jamart J, Vandermeeren Y. Dual-tDCS enhances online motor skill learning and long-term retention in chronic stroke patients. Front Hum Neurosci 2013; 6: 343. DOI: 10.3389/fnhum.2012.00343.
- Sharma R, Aranha VP, Saxena A, Samuel AJ. Effects of dual transcranial direct current stimulation and modified constraint induced movement therapy to improve upper-limb function after stroke: a double-blinded, pilot randomized controlled trial. J Stroke Cerebrovasc Dis 2021; 4: 106227. DOI: 10.1016/j.jstrokecerebrovasdis.2021.106227.
- Wong PL, Yang YR, Tang SC, Huang SF, Wang RY. Comparing different montages of transcranial direct current stimulation on dual-task walking and cortical activity in chronic stroke: double-blinded randomized controlled trial. BMC Neurol 2022; 22: 119. DOI: 10.1186/s12883-022-02644-y.
- Nitsche MA, Liebetanz D, Lang N, Antal A, Tergau F, Paulus W. Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clin Neurophysiol 2003; 114: 2220–2222; author reply 2222-2223. DOI: 10.1016/s1388-2457(03)00235-9.
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982; 14: 377–381.
- Bilney B, Morris M, Webster K. Concurrent related validity of the GAITRite walkway system for quantification of the spatial and temporal parameters of gait. Gait Posture 2003; 17: 68–74. DOI: 10.1016/s0966-6362(02)00053-x.
- Cho KH, Lee HJ, Lee WH. Test-retest reliability of the GAITRite walkway system for the spatio-temporal gait parameters while dual-tasking in post-stroke patients. Disabil Rehabil 2015; 37: 512–516. DOI: 10.3109/09638288.2014.932445.
- Plummer P, Eskes G. Measuring treatment effects on dual-task performance: a framework for research and clinical practice. Front Hum Neurosci 2015; 9: 225. DOI: 10.3389/fnhum.2015.00225.
- Yen CL, Wang RY, Liao KK, Huang CC, Yang YR. Gait training induced change in corticomotor excitability in patients with chronic stroke. Neurorehabil Neural Repair 2008; 22: 22–30. DOI: 10.1177/1545968307301875.
- Murase N, Rothwell JC, Kaji R, Urushihara R, Nakamura K, Murayama N, et al. Subthreshold low-frequency repetitive transcranial magnetic stimulation over the premotor cortex modulates writer’s cramp. Brain 2005; 128: 104–115. DOI: 10.1093/brain/awh315.
- Papegaaij S, Taube W, Hogenhout M, Baudry S, Hortobágyi T. Age-related decrease in motor cortical inhibition during standing under different sensory conditions. Front Aging Neurosci 2014; 6: 126. DOI: 10.3389/fnagi.2014.00126.
- Duncan PW, Propst M, Nelson SG. Reliability of the Fugl-Meyer assessment of sensorimotor recovery following cerebrovascular accident. Phys Ther 1983; 63: 1606–1610. DOI: 10.1093/ptj/63.10.1606.
- Seo HG, Lee WH, Lee SH, Yi Y, Kim KD, Oh BM. Robotic-assisted gait training combined with transcranial direct current stimulation in chronic stroke patients: a pilot double-blind, randomized controlled trial. Restor Neurol Neurosci 2017; 35: 527–536. DOI: 10.3233/RNN-170745.
- Cohen J. A power primer. Psychol Bull 1992; 112: 155–159. DOI: 10.1037//0033-2909.112.1.155.
- McGough JJ, Faraone SV. Estimating the size of treatment effects: moving beyond p values. Psychiatry (Edgmont) 2009; 6: 21–29.
- Nair DG, Renga V, Lindenberg R, Zhu L, Schlaug G. Optimizing recovery potential through simultaneous occupational therapy and non-invasive brain-stimulation using tDCS. Restor Neurol Neurosci 2011; 29: 411–420. DOI: 10.3233/RNN-2011-0612.
- Fusco A, Assenza F, Iosa M, Izzo S, Altavilla R, Paolucci S, et al. The ineffective role of cathodal tDCS in enhancing the functional motor outcomes in early phase of stroke rehabilitation: an experimental trial. Biomed Res Int 2014; 2014: 547290. DOI: 10.1155/2014/547290.
- Lee SH, Kim WS, Park J, Kim J, Paik NJ. Effects of anodal transcranial direct current stimulation over the contralesional hemisphere on motor recovery in subacute stroke patients with severe upper extremity hemiparesis: study protocol for a randomized controlled trial. Medicine (Baltimore) 2020; 99: e19495. DOI: 10.1097/MD.0000000000019495.
- Dodd KC, Nair VA, Prabhakaran V. Role of the contralesional vs. ipsilesional hemisphere in stroke recovery. Front Hum Neurosci 2017; 21: 469. DOI: 10.3389/fnhum.2017.00469.
- Collett J, Fleming MK, Meester D, Al-Yahya E, Wade DT, Dennis A, et al. Dual-task walking and automaticity after Stroke: insights from a secondary analysis and imaging sub-study of a randomised controlled trial. Clin Rehabil 2021; 30: 2692155211017360. DOI: 10.1177/02692155211017360.
- Bijleveld-Uitman M, van de Port I, Kwakkel G. Is gait speed or walking distance a better predictor for community walking after stroke? J Rehabil Med 2013; 45: 535–540. DOI: 10.2340/16501977-1147.
- Corp DT, Youssef GJ, Clark RA, Gomes-Osman J, Yücel MA, Oldham SJ, et al. Reduced motor cortex inhibition and a ‘cognitive-first’ prioritisation strategy for older adults during dual-tasking. Exp Gerontol 2018; 113: 95–105. DOI: 10.1016/j.exger.2018.09.018.
- Ding Q, Triggs WJ, Kamath SM, Patten C. Short intracortical inhibition during voluntary movement reveals persistent impairment post-stroke. Front Neurol 2019; 9: 1105. DOI: 10.3389/fneur.2018.01105.
- McDonnell MN, Stinear CM. TMS measures of motor cortex function after stroke: a meta-analysis. Brain Stimul 2017; 10: 721–734. DOI: 10.1016/j.brs.2017.03.008.
- Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol 1999; 517: 591–597. DOI: 10.1111/j.1469-7793.1999.0591t.x.
- Dimyan MA, Cohen LG. Contribution of transcranial magnetic stimulation to the understanding of functional recovery mechanisms after stroke. Neurorehabil Neural Repair 2010; 24: 125–135. DOI: 10.1177/1545968309345270.
- Park SD, Kim JY, Song HS. Effect of application of transcranial direct current stimulation during task-related training on gait ability of patients with stroke. J Phys Ther Sci 2015; 27: 623–625. DOI: 10.1589/jpts.27.623.
- Aneksan B, Sawatdipan M, Bovonsunthonchai S, Tretriluxana J, Vachalathiti R, Auvichayapat P, et al. Five-session dual-transcranial direct current stimulation with task-specific training does not improve gait and lower limb performance over training alone in subacute stroke: a pilot randomized controlled trial. Neuromodulation 2022; 25: 558–568. DOI: 10.1111/ner.13526.
- Naro A, Billeri L, Manuli A, Balletta T, Cannavò A, Portaro S, et al. Breaking the ice to improve motor outcomes in patients with chronic stroke: a retrospective clinical study on neuromodulation plus robotics. Neurol Sci 2020; 42: 2785–2793. DOI: 10.1007/s10072-020-04875-8.
- Wang RY, Tseng HY, Liao KK, Wang CJ, Lai KL, Yang YR. rTMS combined with task-oriented training to improve symmetry of interhemispheric corticomotor excitability and gait performance after stroke: a randomized trial. Neurorehabil Neural Repair 2012; 26: 222–230. DOI: 10.1177/1545968311423265.
- Sehle A, Stuerner J, Hassa T, Spiteri S, Schoenfeld MA, Liepert J. Behavioral and neurophysiological effects of an intensified robot-assisted therapy in subacute stroke: a case control study. J Neuroeng Rehabil 2021; 18: 6. DOI: 10.1186/s12984-020-00792-1