ORIGINAL REPORT
REHABILITATION WITH INTENSIVE ATTENTION TRAINING EARLY AFTER ACQUIRED BRAIN INJURY PROMOTES BETTER LONG-TERM STATUS ON HEALTH-RELATED QUALITY OF LIFE, DAILY ACTIVITIES, WORK ABILITY AND RETURN TO WORK
Gabriela MARKOVIC, PhD1,2, Aniko BARTFAI, PhD1,2, Marie-Louise SCHULT, PhD1,2 and Jan EKHOLM, MD, PhD1
From the 1Department of Clinical Sciences, Karolinska Institutet, Danderyd University Hospital, Stockholm, 2Division of Rehabilitation Medicine, Danderyd University Hospital, Stockholm, Sweden
Objective: To describe long-term effects on activity, participation, and quality of life (i) at different post-injury starting time points of attention training and (ii) of two different types of rehabilitation with attention training in patients after stroke or traumatic brain injury; and to describe their functioning level.
Design: 2 years after rehabilitation intervention, comparisons were made in one cohort receiving attention training subacute (< 4 months) or post-acute (4–12 months) and in one cohort with two different training methods, a process-based and an activity-based method respectively.
Patients: 100 patients were recruited from our earlier RCT study. They had mild to moderate stroke or traumatic brain injury with relatively limited symptomatology, and all had moderate to severe attention impairment.
Methods: A questionnaire-based interview: EuroQol 5 dimensions, Occupational Gaps Questionnaire, Work Ability Index, self-assessed work status, self-reported employment conditions, sick leave, and experienced cognitive limitations in work performance.
Results: An advantage for patients receiving subacute attention training regarding daily activities, work ability and returning to work.
Conclusion: The results indicate that subacute rehabilitation with attention training (< 4 months) is preferable compared to post-acute intervention (4–12 months). There were only minor differences between the training methods.
LAY ABSTRACT
Previously, in the context of interdisciplinary rehabilitation, we have subjected patients with stroke or traumatic brain injury to additional intensive rehabilitation of attention using two different training methods, a process-based and an activity-based method. The specific attention training commenced either during the first four months after injury or between four and 12 months after injury. In the current study, we report data collected by a telephone interview two years later regarding quality of life and possible limitations in activities of daily life and work. The results show an advantage for patients receiving early rehabilitation regarding daily activities, capacity for work and return to work. Further, there were some minor advantages for patients in the early group, receiving process-based training. Still, the two methods had no significant outcome differences after two years. Thus, the importance of early rehabilitation needs to be considered in organizing rehabilitation services.
Key words: acquired brain injury; cognitive rehabilitation; early rehabilitation; attention dysfunction; attention process training; activity and participation.
Citation: J Rehabil Med 2024; 56: jrm5308. DOI: https://doi.org/10.2340/jrm.v56.5308.
Copyright: © Published by Medical Journals Sweden, on behalf of the Foundation for Rehabilitation Information. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/)
Submitted: Nov 11, 2022; Accepted: Oct 31, 2023; Published: Jan 12, 2024
Correspondence address: Gabriela Markovic, Karolinska Institutet, Department of Clinical Sciences (KIDS), Division of Rehabilitation Medicine, Danderyd Hospital, SE-182 88 Stockholm, Sweden. E-mail: gabriela.markovic@ki.se
Competing interests and funding: The authors have no conflicts of interest to declare.
Attention difficulties are common cognitive sequelae of acquired brain injury (ABI) (1, 2). Attention supports other cognitive functions and, as such, it is a core component of cognitive skills underlying human activities. Small changes in attention might significantly impact a person’s daily life by affecting learning skills, daily functioning, and work (3, 4).
There are a variety of approaches for rehabilitation of attention impairments depending on injury severity and the specific nature of the attention impairment, with interventions that are either restorative, designed to improve underlying cognitive processes, or compensatory, improving performance and allocating attention resources (5–7). One of these approaches is systematic, hierarchical training with attention process training (APT) (8). This method targets the different aspects of attention, i.e., focused, sustained, selective, alternating, and divided into tasks of increasing difficulty and complexity. APT is a successful method post-ABI for adults (9–11) and children (12). APT is recommended in several guidelines (6, 7) and is considered an evidence-based method in the chronic stage after ABI. Most studies report these favourable effects from a post-intervention perspective. In contrast, long-term studies are scarce (4, 13), with only 2 studies focusing on the long-term effect of APT intervention in terms of strategy use (14) and maintenance of functional level (15). Therefore, the current study focuses on long-term aspects.
Activity-based attention training (ABAT) is a performance-skills and metacognitive strategy training emphasizing the importance of conscious cognitive processes in initiating, performing, and controlling attention-demanding activities, such as cooking and studying (5, 10, 16, 17).
Previously, we conducted a series of RCT studies investigating the short-term effects of attention training within the first year after ABI (18), comparing the effects of APT and ABAT in combination with an interdisciplinary inpatient and outpatient rehabilitation programme in 2 cohorts (18–20). Patients from the 2 largest diagnostic groups with ABI, stroke, and traumatic brain injury (TBI) were included in the study, following the same inclusion and exclusion criteria. The results of this short-term rehabilitation suggested an advantage for patients receiving APT (20–22). The current study examines long-term effects of the choice of rehabilitation intervention.
The definition of time intervals for rehabilitation of subacute ABI, compared with chronic ABI, varies, but the first year after ABI is usually considered an early stage (23). However, the path of spontaneous recovery of cognitive functions after ABI is described as non-linear during that timeframe, with different physiological mechanisms and a steeper curve during the first 3–4 months (24). Consequently, data were collected from 2 cohorts (18) since, during the past decade, several studies have reported positive effects of subacute cognitive rehabilitation (< 3 months) after ABI (25–32). These studies reported substantial changes during inpatient rehabilitation. Although effect sizes subsided over time, significant improvements were maintained up to 3 months after discharge. Thus, the choice of the starting time-point for attention-improving interventions is relevant.
People of working age with stroke or TBI are expected to live with a lifelong disability affecting both well-being and health-related quality of life (HRQoL). Active coping strategies (33) and return to work (RTW) have proved to be of importance for well-being after ABI (34–36), while cognitive impairment is associated with worse HRQoL (37) and attention dysfunction specifically (38–41) more likely results in failure to RTW. The possibility of RTW (42) depends not only on medical and psychological pre-and post-injury factors (43), but also on workplace-related circumstances (44). Several studies have highlighted the importance of investigating the long-term effects of cognitive rehabilitation (4, 36, 45, 46) along with the need to measure these changes in terms of changes in activity and participation (47), since those measures are assumed to reflect closer real-life changes in, for example, personal independence and work situation (48). With this background, the current study examines the patients’ working ability and RTW 2 years after rehabilitation.
This study describes the activity status, participation, perceived work ability, and HRQoL in participants (18) receiving intensive attention training within the first year post-ABI. The study aimed to compare the long-term effects of: (i) subacute start of attention training with post-acute start; and (ii) 2 different attention interventions, process-based and activity-based. Based on our previous demonstration of an advantage of APT training within 4 months post-injury (21, 49) and a positive effect on work-performance within 4–12 months post-injury (20), one of the current study hypotheses was that the long-term effectiveness of APT training would be greater than that of the ABAT form of attention training in patients studied 2 years after the initial training.
METHODS
Study design
Participants from the previous RCT study (ClinicalTrials.gov.trial registration NCT02091453) (18, 50) were recruited 2 years after the initial rehabilitation intervention. Comparisons were made in 1 cohort that had undergone attention training either subacute (SA) (< 4 months post-ABI) or post-acute (PA) (4–12 months post-ABI) and in a second cohort that had undergone 1 of 2 different training methods, a process-based method (APT) and an activity-based method (ABAT).
Participants
The participants had had either a mild-to-moderate stroke or TBI with relatively homogenous symptomatology, no aphasia, psychiatric symptoms, or neglect. Satisfactory levels of logical reasoning, memory, and fine motor functions were required to participate in the APT programme. Attention impairment was moderate to severe, measured by the APT test (8) preceding the attention training. Demographic characteristics are shown in Table I. Injury-related characteristics (lesion localization and distribution) are shown in Table II: a rehabilitation physician and a neuropsychologist classified lesion distribution and related features.
| Variable | Total sample |
| Age, years, mean (SD)/median | 49 (10)/52 |
| Age range, n (%) | |
| 19–29 years | 6 |
| 30–49 years | 36 |
| 50–64 years | 58 |
| Sex, female, n | 49 |
| Marital status, n | |
| Married/co-habitant | 76 |
| Single | 22 |
| With parents | 2 |
| Educationa, n | |
| ≤ 12 years | 31 |
| 13–15 years | 44 |
| > 16 years | 25 |
| Employment (time of injury), n | |
| Working or studying | 81 |
| Not working | 19 |
| aCompleted years of education, from elementary school to higher education. | |
| SD: standard deviation. | |
| Variable | Total sample |
| Aetiology, n | |
| Strokea | 79 |
| Traumatic brain injuryb | 21 |
| Lesion side, n (%) | |
| Left/right hemisphere | 42/31 |
| Bilateral | 27 |
| Lesion distribution, n (%) | |
| Focal/multifocal (≥ 2) | 47/53 |
| Lesion localisation, n (%) | |
| Anterior | 25 (29) |
| Posterior | 16 (19) |
| Subcortical | 35 (41) |
| Global | 9 (11) |
| aOf which strokes 61% were thrombosis, 27% haemorrhage, 10% subarachnoid haemorrhage, and 2% thrombosis and haemorrhage. bEight participants had haematoma, 4 had contusions, and 9 had both haematoma and contusions. Traumatic brain injury was a result of traffic accidents (n = 9), falls (n = 8), sports (n = 3) and assault (n = 1). | |
Procedure
An experienced research nurse, who was unfamiliar with the participants and blinded to intervention, contacted the participants, and performed data collection by telephone. All participants (n = 120) from the previous RCT (18) were contacted by letter and a subsequent telephone call. Of these, 16 did not respond, 3 did not have a contact address, and 1 was deceased; therefore, the final number of respondents was 100. After signing informed consent, the participants received the questionnaires by post, and the research nurse interviewed them within 1 week.
Outcome assessments
The current study selected outcome measures (51) guided by a philosophy that outcome research in ABI should focus on function and participation in daily life. This study describes the status of the participants in relation to a healthy reference group according to each measure’s standard outcome.
Health-related quality of life EuroQol-5 dimensions questionnaire (3L). EQ-5D, a generic, patient-reported HRQoL instrument, consists of 5 dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) and 3 severity levels (no problems, moderate problems, severe problems) (52). A single-index value can be derived for each dimension. The dimensions are converted into an index value (−0.594 and + 1.000) (53), anchored at 1 (total health) and 0 (dead). Swedish normative health index is 0.898 for men and 0.886 for women (54). The EQ-5D is a valid measure of quality of life (QoL) after stroke (55).
Occupational Gaps Questionnaire. The OGQ, version 1.1, measures perceived participation in everyday occupations (56). An occupational gap (OG) occurs when the individual: (i) does not perform an activity that they want to (OG 1); or (ii) performs an activity that they do not want to (OG 2). This study considers only OG 1. OGs were examined for 28 activities, including 8 instrumental ADLs, 6 social activities, 10 leisure activities and 4 work-related activities. Higher scores correspond to higher restrictions. The scale is dichotomous, distinguishing situations with no OG from conditions with OGs. The results are presented regarding the distribution of OGs in relation to a healthy reference group (56).
Work ability. The Work Ability Index (WAI) (57) measures work ability in 7 dimensions: current work ability (WAS) compared with lifetime best (score 0–10), work ability concerning job demands (score 2–10), number of medical diagnoses (score 1–7), impaired work performance (score 1–6), sickness absence in the last 12 months (score 1–5), expected work ability in the forthcoming 2 years (score 1–7), and mental resources (score 1–4). Scores are summed with (range 7–49) and without the diagnosis list (range 6–42). High numbers indicate better self-reported work ability. The number of diagnoses was based on medical records at inclusion. Results are presented in relation to a healthy reference group (58). The total WAI score can be grouped into 4 classes of work ability: 1: “poor” (need to restore); 2: “moderate” (need to improve); 3: “good” (need to support); and 4: “excellent” (need to maintain) (59). Self-rated work ability score (WAS) is based on the first question: “Current work ability compared with the lifetime best” (0 = complete work disability, 10 = best work ability). Previous studies demonstrate a strong association between WAS and the complete WAI. Therefore, WAS has been recommended as a simple, reliable indicator of work ability (60).
Self-reported employment conditions, sick leave, and experienced cognitive limitations in work performance. A structured questionnaire developed by the research group covered self-reported employment conditions, sick leave, and experienced cognitive limitations in work performance. Eight questions concerning employment conditions could be answered with “yes” or “no”. Nine options were presented for the nature and extent of the actual work situation and sickness allowance/sickness compensation (0, 1/4, ½, ¾, or total compensation). The participants could select more than 1 option. Questions (n = 17) concerning experienced limitations in work performance due to the ABI could be answered with “yes”, “sometimes”, and “no”. The questions focused on cognitive and behavioural difficulties in work due to attention dysfunction, such as concentration demands, understanding and completing work tasks, forgetfulness, error-proneness, problems initiating and structuring work tasks, and the need for technical and emotional support.
Previous interdisciplinary rehabilitation and attention training
All subjects in the current study had participated in intensive (6 h/day, 4–5 day/week for 8–12 weeks) interdisciplinary rehabilitation, which was completed 2 years prior to the start of the current study. The rehabilitation was provided by rehabilitation physician, nurse, physiotherapist, occupational therapist, neuropsychologist, speech and language therapist, and social worker. Cognitive interventions were based on the work of the Cognitive Rehabilitation Task Force (CRTF) of the American Congress of Rehabilitation Medicine (10, 61). In addition to the regular rehabilitation, the participants received a total of 20 h of attention training, 3–5 times/week, for 5–6 weeks. The attention training was randomized into APT or ABAT.
The APT (8) is a process-oriented, theoretically and hierarchically based, individualized attention-training programme considered a “practice standard” treatment for attention deficits after brain injury (10, 61). It comprises repetitive exercises with increased difficulty and meta-cognitive strategy training for improved and more flexible use of strategies in daily life (generalization), insight, and motivation. The APT is a direct structured neuropsychological intervention. A neuropsychologist performs the training session individually (45–90 min/session), improving performance on training tasks and immediate measures of global attention (10).
The ABAT is an occupational therapy intervention comparable to the Cognitive Orientation to Occupational Performance (CO-OP) (62, 63) aiming at functional skills training on activity level and metacognitive strategies to improve performance on trained tasks. An occupational therapist conducted ABAT involving attention-demanding everyday activities in personal care, household activities, work, leisure, and social activities (60–120 min/session) (20).
Statistical analysis
Pearson χ2 test was used to analyse sex, marital status, education, and injury-related data. A parametric t-test was used to compare groups on age, timing and length of intervention, the timing of follow-up, results of psychometric testing, and level of attention dysfunction. Descriptive statistics (frequencies, mean, median, standard deviations, percentiles, and confidence intervals) were calculated for the telephone interview questionnaires. Group comparison was analysed with the Pearson χ2 test. Statistical differences between groups and subgroups were analysed for self-rated health expressed in EQ-5D dimensions (Pearson χ2) and EQ-5D index (Mann–Whitney U test).
For the OGQ, this study presents data on OG 1 (i.e., “does not perform an activity that he/she wants to”). The proportion of reported OGs in the study population was compared with a Swedish age-matched reference population of 811 persons (56).
Between-group comparisons were made for the start of attention training, subacute group (SAG) vs post-acute group (PAG) and type of intervention (APT vs ABAT), as well as combined for the start of attention training and type of intervention (SAG-APT, SAG-ABAT, PAG-APT, PAG-ABAT). One-way analysis of variance (ANOVA) and Tukey-Kramer post hoc analyses were used to compare subgroups for all outcome measures. Effect sizes (ES) (64) were calculated according to Cohen’s d (ES D) (small = 0.2, medium = 0.5, large = 0.8). Levene’s Test for Equality of Variances measured the homogeneity of variances within groups. The statistical significance level was set at p ˂ 0.05 2-tailed for all analyses. IBM Corp. Released 2013. IBM SPSS Statistics for Windows version 22.0 Armonk, NY: IBM Corp. was used for statistical analysis.
Ethics
The study was approved by the Karolinska Ethics Committee (2014/1270-32) and was performed according to the principles of the Declaration of Helsinki.
RESULTS
No differences were found between the SAG and PAG groups, nor between APT and ABAT regarding age, marital status, education, employment, or injury-related characteristics. There was a sex difference, with more men in the SAG group than the PAG group (Pearson χ2 5.725, df = 1, p = 0.017) and more women in the APT group than the ABAT group (Pearson χ2 4.944, df = 1, p = 0.026). The initial APT test performance was lower for PAG than for SAG (t(98) = 3.367, p = 0.001). No differences in the APT test were found between the APT and ABAT groups. No impacts of sex and performance on the APT test were found on analysis of co-variance.
Health-related quality of life (EuroQol 5 dimensions)
There were no statistically significant differences in EQ-5D index values between SAG and PAG groups nor between APT and ABAT (Mann–Whitney U test) groups. Equal variances within groups are not assumed (F(3,96) = 3,60, p = 0.016) with SAG-APT showing less within-group variability (t(52) = 2.120, p = 0.039). The EQ-5D index values for the subgroups according to timing and type of intervention are shown in Fig. 1. SAG-APT had the highest mean HRQoL (0.80) and PAG-APT had the lowest (0.66).
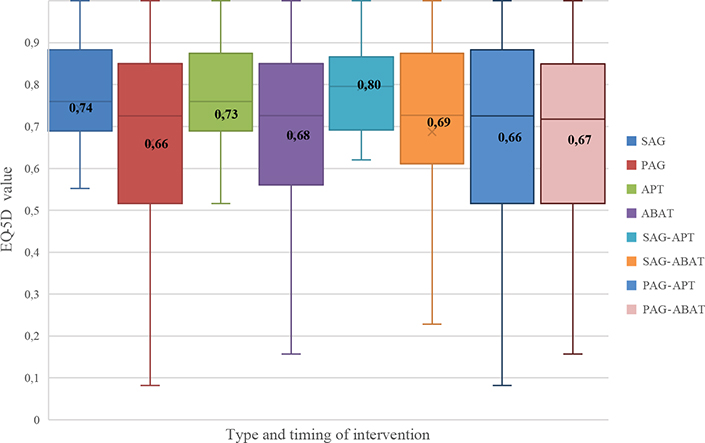
Fig. 1. EuroQol 5 dimensions (EQ-5D) index values for timing and type of intervention. Boxplots represent the distribution between the first and third quartile within each group, with the median as centreline and inserted mean values. Swedish reference data for EQ-5D index values in mean (standard deviation; SD) for a healthy population (n = 25,867; aged 30–104 years) is 0.898 (0.112) for men, and 0.886 (0.116) for women (54). SAG (sub-acute group), PAG (post-acute group), APT (attention process training), ABAT (activity-based attention training).
Examination of differences regarding type and timing for the individual variables in EQ-5D indicated that participants from SAG reported fewer problems doing usual activities, χ2 (1, N = 100) = 6.578, p = 0.016). Eighty-eight percent of the participants did not have problems with self-care, and 60% of the group reported an HRQoL corresponding to a healthy population. Proportions of participants reporting problems in the 5 EQ-5D dimensions are shown in Fig. 2.
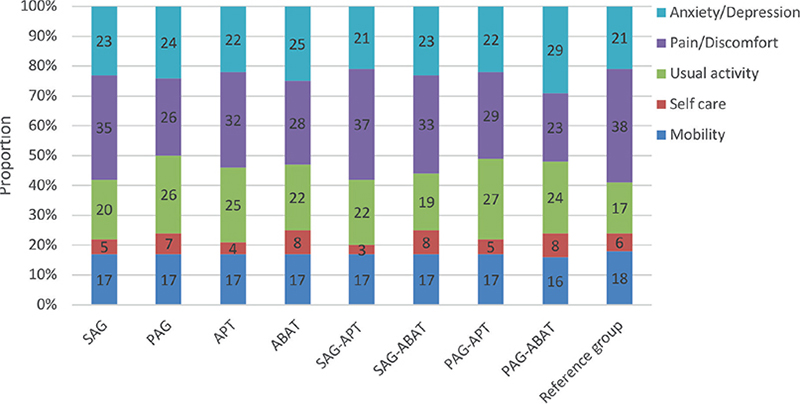
Fig. 2. Profile of proportions of participants (%), n = 100, reporting problems in the 5 EuroQol 5 dimensions (EQ-5D) dimensions. Groups and subgroups of timing and type of intervention are indicated, and a reference group of healthy general population, n = 25,867 (54). SAG (sub-acute group), PAG (post-acute group), APT (attention process training), ABAT (activity-based attention training).
Occupational Gaps Questionnaire
The number of OGs varied between the 4 subgroups (Table III). In the SAG group, there were significantly more participants with no OGs (t(98) = 2.008, p = 0.047) than in the other 3 subgroups, including reporting fewer OGs (t(98) = –2.531, p = 0.031) than the median of an age-corrected healthy population. Conversely, PAG (t(98) = –2.252, p = 0.027) and PAG-APT groups reported significantly more OGs (F(3,98) = –2.054, p = 0.045) than other subgroups. There was no significant difference in OGs reported by the APT and ABAT groups.
| Occupational gaps (OGs)* | Timing of intervention | Type of intervention | Type and timing of intervention | |||||
| SAG n (%) | PAG n (%) | APT n (%) | ABAT n (%) | SAG-APT n (%) | SAG-ABAT n (%) | PAG-APT n (%) | PAG-ABAT n (%) | |
| Participants with no gaps | 7 (13) | 4 (9) | 8 (15) | 3 (7) | 5 (19) | 2 (8) | 3 (11) | 1 (5) |
| Participants with 1 OG up to the median number of gaps | 23 (43) | 11 (23) | 22 (41) | 15 (32) | 12 (44) | 12 (42) | 7 (26) | 4 (20) |
| Participants with greater than the median number of gaps | 23 (43) | 32 (68) | 24 (44) | 28 (61) | 10 (37) | 13 (50) | 17 (63) | 15 (75) |
| *Median number of OGs in the reference population were age 20–29 years = 5 OGs, age 30–49 years = 4 OGs, age 50–64 years = 2 OGs, age > 65 years = 1 OG. For the reference population as a total, median number of OGs = 3. | ||||||||
| SAG: sub-acute group; PAG: post-acute group; APT: attention process training; ABAT: activity-based attention training. | ||||||||
The same frequency pattern of reported OGs (Fig. 3) was found in Instrumental ADL, Leisure activities, Social Activities, and Work- or work-related activities between SAG and PAG groups. The same was true for APT and ABAT groups. The highest frequency of OGs concerned cleaning, performing heavy household tasks, cultural activities and reading. Fewer problems were reported in grocery shopping, transport, meeting relatives and friends and taking care of and raising children. The SAG group reported fewer OGs in the domain of Leisure activities: “Participating in sports” (t(1) = 5.08, p = 0.024) and “Reading a newspaper” (t(1) = 3.348, p = 0.004) as compared to other subgroups. No other differences were found between study arms. The distribution of OGs in order of prevalence for all participants is shown in Appendix S1.
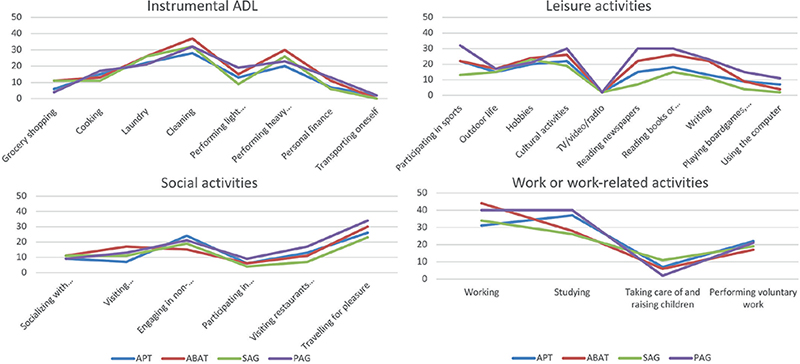
Fig. 3. Frequency of reported occupational gaps (limitations in activity) in the Occupational Gaps Questionnaire in the different domains of activities/participations for type and timing of intervention, respectively. Distribution is presented in percentage. Low percentage signifies a better functional level. SAG: sub-acute group; PAG: post-acute group; APT: attention process training; ABAT: activity-based attention training.
Self-reported employment conditions
The SAG group (Pearson χ2 = 11.926, df = 1, p = 0.001) reported a higher prevalence (47%) of successful RTW, defined as gainful employment at ≥ 75%, compared with the PAG group (22 %). The SAG-ABAT group reported successful RTW (Pearson χ2 = 13.354, df = 3, p = 0.003), compared with subgroups. There were no differences in RTW between APT and ABAT.
Employment conditions for the subgroups are shown in Table IV. The proportion of participants reporting working at the same work having the same or adapted work tasks varied greatly between subgroups (35–74%), with more participants in SAG, APT and SAG-APT groups, respectively, reporting the same or adapted work (63–74%). Fourteen participants reported having no gainful employment, and 17 had changed jobs due to health reasons.
| Variable | Timing of intervention | Type of intervention | Type and timing of intervention | |||||
| SAG n = 53 n (%) | PAG n = 47 n (%) | APT n = 54 n (%) | ABAT n = 46 n (%) | SAG-APT n = 27 n (%) | SAG-ABAT n = 26 n (%) | PAG-APT n = 27 n (%) | PAG-ABAT n = 20 n (%) | |
| Actual work situation | ||||||||
| Employed or self-employed | 32 (60) | 21 (45) | 28 (52) | 25 (54) | 16 (60) | 16 (62) | 12 (44) | 9 (45) |
| Employee 75–100%a | 25 (47) | 7 (22) | 16 (30) | 16 (35) | 11 (41) | 14 (54) | 5 (19) | 2 (10) |
| Not in gainful employment | 5 (9) | 9 (19) | 8 (15) | 6 (13) | 2 (7) | 3 (12) | 6 (22) | 3 (15) |
| Work retraining | 2 (4) | 2 (4) | 5 (9) | 5 (11) | 2 (7) | 6 (23) | 4 (15) | 5 (20) |
| Working at the same work (same work tasks) | 21 (40) | 15 (32) | 23 (43) | 13 (28) | 13 (48) | 8 (31) | 10 (37) | 5 (20) |
| Working at the same work (adapted work tasks)b | 14 (26) | 6 (13) | 11 (20) | 9 (20) | 7 (26) | 7 (27) | 4 (15) | 2 (10) |
| Changed job due to health reasons | 7 (13) | 10 (21) | 6 (11) | 11 (24) | 1 (4) | 6 (23) | 5 (19) | 5 (20) |
| Sick benefit or activity compensation | ||||||||
| No sickness benefit or activity compensation | 44 (83) | 35 (74) | 47 (87) | 32 (70) | 24 (89) | 23 (88) | 20 (74) | 12 (60) |
| Sickness benefit or activity compensation 25–50% | 1 (2) | 5 (12) | 4 (7) | 2 (8) | 1 (4) | 2 (8) | 0 (0) | 5 (20) |
| Sickness benefit or activity compensation ≥ 75% | 7 (15) | 6 (13) | 3 (5) | 10 (22) | 2 (7) | 1 (4) | 5 (26) | 5 (20) |
| aParticipants (n = 5) working at 75% also receive activity compensation at 25%. bParticipants on work retraining (n = 17) are included in this group. | ||||||||
| The participants could select more than 1 option in the questionnaire. | ||||||||
| SAG: sub-acute group; PAG: post-acute group; APT: attention process training; ABAT: activity-based attention training. | ||||||||
Forty-six percent of all participants self-reported receiving sickness benefits or sickness compensation to some degree, 70% of whom (n = 33) received full payment due to disability. In Sweden, if a person has an RTW less than full-time, they can receive sickness compensation for the remainder up to 100%. No significant difference existed between groups and subgroups for the self-reported degree of sick leave, sickness allowance or sickness compensation.
Work Ability Index
The mean WAI Total score for the different subgroups varied between 29 and 33 points, corresponding to moderate work ability, implying a “need to improve” work ability.
SAG reported better work ability (WAI Total Score; independent sample Mann–Whitney U test, p = 0.00) with a strong ES D (Cohen’s d = –0.92) and higher self-rated work ability (WAS = t(92)3,338, p = 0.001) (Table V) than PAG.
| Reference* n = 1,786 Mean | Timing of intervention | Type of intervention | |||
| SAG (n = 53) Mean (SD)/md | PAG (n = 47) Mean (SD)/md | APT (n = 54) Mean (SD)/md | ABAT (n = 46) Mean (SD)/md | ||
| WAI Total Score (n = 74)1 | 41.53 | 36 (8)/38 | 29 (9)/29 | 33 (9)/33 | 33 (9)/34 |
| Individual resources | |||||
| a. Current WA compared with estimated best (WAS) | 8.25 | 6 (3)/8 | 4 (3)/5 | 5 (3)/6 | 5 (3)/6 |
| b1WA in relation to physical demands (current work) | 4.29 | 4 (1)/4 | 4 (1)/4 | 4 (1)/4 | 4 (1)/4 |
| b2WA in relation to mental demands (current work) | 4.23 | 4 (1)/4 | 3 (1)/4 | 4 (1)/4 | 4 (1)/4 |
| f. Own prognosis of WA (2 years) | 6.64 | 6 (2)/7 | 5 (2)/7 | 5 (2)/7 | 6 (2)/7 |
| g. Enjoying daily activities (mental resources) | 2,9 | 3 (1)/3 | 3 (1)/3 | 3 (1)/3 | 3 (1)/3 |
| WAI Individual Health Factor2 | |||||
| c. Number of medical diagnoses | 5.82 | 4 (1)/4 | 3 (1)/3 | 4 (1)/4 | 4 (1)/4 |
| d. Estimated WA impairment due to diseases | 5.24 | 3 (1)/3 | 2 (1)/2 | 3 (1)/3 | 2 (1)/3 |
| e. Sick leave (last 12 months) | 4.15 | 3 (1)/3 | 3 (1)/2 | 3 (2)/2 | 3 (2)/2 |
| 1Total score for the WAI dimensions a, b1, b2, f, g; 2Total score for WAI dimensions c, d, e; *Values of reference from a healthy general population (58). | |||||
| SAG: sub-acute group; PAG: post-acute group; APT: attention process training; ABAT: activity-based attention training; SD: standard deviation; md: median. | |||||
There were no differences in the WAI Total Score or the WAS, depending on the interventions APT or ABAT only. However, SAG-APT reported better outcome on the WAI Total Score (F(3,76) = 4,755, p = 0.004) and the WAS (F(3,90) = 3,802, p = 0.013).
The total WAI score (Table VI) grouped the participants into 4 classes, which showed that SAG (62%) self-assessed their work ability to be “good” or “excellent” to a higher degree than PAG (19%) (Fisher’s χ2 test, p = 0.001; post hoc independent-sample Mann–Whitney U test, p = 0.000). No statistical differences were found between the APT and ABAT groups. When comparing the subgroups, the SAG-APT group reported a higher self-assessed level of work ability (t(3) = 4.996, p = 0.003).
Experienced limitations in work performance
Participants in the different groups reported cognitive and behavioural problems (Table VII), the most frequent issues concerned being more easily disrupted at work, more easily getting tired, experiencing work tasks taking longer, and concentration problems at work. The PAG group reported more problems with work tasks taking longer time than expected (Pearson χ2 = 10.361, df = 2, p = 0.006). Participants receiving the APT reported fewer problems due to forgetting things at work (Pearson χ2 = 6.428, df = 2, p = 0.040) and keeping the workplace tidy (Pearson χ2 = 6.887, df = 2, p = 0.032). The SAG-APT group (t(3) = 15.910, p = 0.013) reported fewer problems keeping their workplace tidy.
All study participants experienced support from their co-workers, and 92% reported having supportive managers.
DISCUSSION
An important finding was that participants starting attention training within 4 months post-ABI reported significantly higher outcomes on HRQoL and work participation and fewer activity limitations. However, only minor differences were found for a specific intervention programme, favouring the advantage of APT within 4 months post-injury.
The results strengthen earlier findings concerning the importance of cognitive rehabilitation within the first year after ABI for functional outcome (27, 28, 32), psychological well-being (30), and vocational functioning (29, 65). Our data strongly support cognitive rehabilitation within the first 3–4 months after ABI for patients with attention impairment.
Furthermore, the subacute group perceived fewer restrictions in everyday occupations (43% vs 68%). No study was found in the literature review for the current study comparing a subacute vs a post-acute intervention group on the results of limitations in daily occupations. The time intervals between rehabilitation and follow-up in existing studies, deviate too much from our study to enable comparisons (56, 66, 67, 68).
The subacute and post-acute groups followed the same pattern when reporting restrictions in different daily occupations (Fig. 3). However, our subacute group reported fewer restrictions for “hobbies” (15%) in contrast to our post-acute group (32 %) and to Bergstrom’s study (32%) (68). The results showed that sub-acute cognitive rehabilitation resulted in higher self-rated work ability (e.g., index value), especially for those receiving APT. Work ability seems linked to functional impairments, attitudes towards disability, and motivation to work (69) and depends on the person’s insight into injury-related problems that might influence work performance (70). In an earlier study on the management of attention (14) we found that increased self-awareness, paired with coping strategies at an early stage, potentially mitigates expedience in performance despite disability and perceived limitations in activity, which could account for the findings of higher WAI for the APT group. However, we can only hypothesize why the impact seems greater with sub-acute rehabilitation.
The comparisons showed a higher prevalence of successful RTW in patients receiving sub-acute intervention than post-acute intervention. RTW is essential for QoL (34) and is a realistic goal for many working-age patients with ABI. Thus, RTW rates are of interest in rehabilitation research, even if the interpretation beyond the usual individual functioning level needs to be careful since societal factors influence it to varying degrees. When comparing RTW rates in studies from different countries, comparisons need to respect the differences in each country. The RTW rate of 47% for gainful employment ≥ 75% is well within the range of earlier reports (43, 71–73). No study has been found comparing the sub- vs post-acute attention-training long-term outcome on RTW. Regarding RTW, the current study results do not support selecting one training method above the other. Cognitive function (38, 74–76), independence in ADL, together with pre-injury factors, such as education and type of job, and perceived work ability (76) are identified as predictive factors (75, 77) for successful RTW. Although attention dysfunction has proven to have a significant impact on RTW within 18 months post-onset (38), studies identifying the potential impact of specific cognitive disability on RTW (78) or outcome of RTW post rehabilitation (74) are scarce. No study has been found comparing the 2 attention-training methods’ effects on long-term RTW.
In the current study, there were only minor statistical differences between the 2 attention interventions using measures of activity and participation, which was unexpected. Previously, our research group reported the advantage of APT on a neuropsychological outcome measure, Paced Auditory Serial Addition Test (21), using a statistical process method (49). On the level of activity and participation, using a standardized office-work task and evaluated according to the Assessment of Work Performance measure (AWP) (79, 80), significant differences were found, favouring APT post-intervention and at a 3-month follow-up for some process skills (Mental Energy, Knowledge, Temporal Organization, Adaptation and Physical Energy) (20). AWP assesses an individual’s observable skills during work performance, i.e., how efficiently and appropriately a client performs a work activity. These skills are subserved by executive and attention functions trained in the programme, suggesting some transfer effects of the training from body to activity levels. For other outcome measures, such as the WAI, a significant improvement was observed after intensive rehabilitation of attention. However, there were no differences between the 2 training methods, as observed in the current study for WAI.
It should be noted that the minor differences observed in the current study supporting APT, and particularly in the subacute group (SAG-APT has the highest QoL, highest successful RTW, better outcome on WAI Total Score and fewer problems in organizing their workplace). In addition, APT patients reported fewer memory problems at work. The only discrepant result was significantly more OGs for PAG-APT.
The results of the current study can be interpreted in several contexts: (i) differences between process-based and activity-based attention training might disappear gradually. As time passes, practical behavioural elements, strategies and skills acquired in activity-based training get individually incorporated into everyday behaviour according to situations, needs and preferences. This slow process might smooth out early differences in the effects of training methods in the long-term. Patients utilize rehabilitation programmes differently due to their strengths and weaknesses, leading to a gradual absorption of the newly learned behavioural elements or performance skills in daily life. This process makes the effects of underlying cognitive training in one area (attention) challenging to discern. In our earlier interview study with participants receiving a multidisciplinary rehabilitation programme and APT (14), we provided examples of how patients apply APT training after discharge from rehabilitation. (ii) Conceptual differences in measures may be another reason for the different results. Earlier studies found low to moderate correlations between measures for cognitive tests (body-function level) and the Assessment of Motor and Process Skills (AMPS) activity level (81). As discussed previously (80), some outcome measures on activity level, such as QoL and work ability, are global measures primarily assessing the impact of a disease without referring to specific behaviours. The effects of a directed cognitive intervention might not be easily detected in such a context.
Another category of outcome measures on activity level is performance skills, defined as small observable units of behaviour used to perform a specific task (82) and offer higher conceptual proximity to variables in a neuropsychological test situation. However, these behavioural units, such as time management and structuring one’s workstation, require attentional support to varying extents. Improvements in attention, a cognitive function, are thus differentially reflected in activity changes as a function of task requirements and individual qualifications. Furthermore, these measurement instruments, such as AMPS and AWP, allow the selection of different tasks or activities. Hence, differences in types of activities make group comparisons more problematic.
An additional group of activity-based measures are functionally based standardized performance-based instruments directly relevant to clinically meaningful outcomes (6). Our standardized office-task targeting skills in attention, organization, and processing skills is an example of this approach (80). The task follows a formalized procedure with standardized scoring according to predefined criteria and values for a healthy comparison group.
Due to earlier criticism regarding the lack of evidence in the generalization of cognitive training to real-life activities (83) and to answer the calling of earlier reviews (84, 85) we have selected outcome measures on the level of activity and participation in the current study. Our varying results suggest a need to distinguish between different subcategories and choose measures closely related to the target of rehabilitation, i.e., performance-based measurements, as recommended (6).
When relating the current study results to healthy reference groups for each measure, the results indicate that the subacute group rates their QoL at the same level as the healthy population and has few restrictions in daily occupations. However, participants in the current study rated their work ability (mean WAI total score) as “moderate” work ability (need to improve) in contrast to a healthy reference group ranking theirs as “good” work ability (59). The lower ratings may be interpreted as that, after 2 years, the patients still needed measures to improve their work ability, especially the 26% of the participants who experienced poor work ability.
Study limitations
First, due to the recommendation for homogeneous groups (50) the current study participants showed a restricted range of clinical symptoms, excluding patients with comorbidities, including higher severity of injury and aphasia. The current results are based on patients with mild to moderate deficits post-ABI, so the results do not apply to patients with more severe injuries and complexity in cognitive impairment.
The dropouts from the current study had lower results on logical thinking at the time of intervention, which we consider having minimal influence on the conclusions.
These results are based on self-report data regarding the working situation and social support. A third limitation thus concerns the choice of measures, as self-reports are not always reliable, due to the complexity of these measures. Independent data sources, such as registered data from insurance agencies, are recommended to give as correct data as possible on sick leave days and sickness compensation extent and periods. However, no such resources were available. A strength of the current study was the telephone-based follow-up for completing the reports, circumventing the most common confounders in self-reports, i.e., missing data and the influence of next-of-kin.
Conclusion
This study followed up working-age patients on daily activities, RTW and work ability 2 years after they had undergone intensive attention training for moderate attention impairment post-ABI. The results emphasize the importance of subacute (≤ 4 months) over post-acute (4–12 months) start of rehabilitation. When comparing attention training interventions, no differences could be distinguished except for a few minor advantages that could be demonstrated for APT in the group who underwent subacute rehabilitation. Valid measurements of how impaired functions influence activities and participation in the long term are complex and require further research.
Clinical implications
The results of this study provide information concerning long-term outcomes of subacute intensive attention training regarding QoL and aspects of working capacity for patients with ABI of working age. These results contribute to the accumulating body of knowledge concerning the importance of early rehabilitation. According to current clinical practice, patients are mobilized and activated during the acute inpatient and rehabilitation phases and then, after housing adaptation, often discharged to home-based rehabilitation. However, the resources of the teams providing home-based rehabilitation are not sufficient to meet the requirements of intensive attention training. These results indicate the need for providing more patients with shorter periods of intensive sub-acute outpatient rehabilitation.
Differences between training methods seemed to attenuate over time. However, the clinician should note that both APT and ABAT were accompanied by extensive metacognitive training and tasks focusing on attention-demanding activities, as recommended by several guidelines (6, 7, 45).
ACKNOWLEDGEMENTS
The study was funded by Promobilia Foundation, The Swedish Research Council, The Swedish Association for Survivors of Polio, Accident, and Injury (RTP), The Swedish Stroke Association, ALF agreement for clinical research, and supported by the Department of Rehabilitation Medicine, Danderyd Hospital. The authors acknowledge the assistance of research nurse Seija Lund in data collection, and medical statistician Fredrik Johansson in data analysis. We are grateful to the patients who have shown a willingness to participate. The content is solely the responsibility of the authors.
REFERENCES
- Ponsford J, Bayley M, Wiseman-Hakes C, Togher L, Velikonja D, McIntyre A, et al. INCOG recommendations for management of cognition following traumatic brain injury, part II: attention and information processing speed. J Head Trauma Rehabil 2014; 29: 321–337.
- Barker-Collo S, Feigin V, Lawes C, Senior H, Parag V. Natural history of attention deficits and their influence on functional recovery from acute stages to 6 months after stroke. Neuroepidemiol 2010; 35: 255–262.
- Söderback I, Ekholm J, Caneman G. Impairment/function and disability/activity 3 years after cerebrovascular incident or brain trauma: a rehabilitation and occupational therapy view. Int Disabil Stud 1991; 13: 67–73.
- Virk S, Williams T, Brunsdon R, Suh F, Morrow A. Cognitive remediation of attention deficits following acquired brain injury: a systematic review and meta-analysis. NeuroRehabilitation 2015; 36: 367–377.
- Sohlberg MM, CA Cognitive Rehabilitation: an integrative neuropsychological approach. New York: The Guilford Press; 2001.
- Cicerone KD, Dams-O’Connor K, Eberle R, Ganci K, Langenbahn DM, Shapiro-Rosenbaum A, et al. The ACRM cognitive rehabilitation manual & textbook. translating evidence-based recommendations into practice. Second edition. Reston, VA, USA ACRM Publishing; 2022.
- Ponsford J, Velikonja D, Janzen S, Harnett A, McIntyre A, Wiseman-Hakes C, et al. INCOG 2.0 guidelines for cognitive rehabilitation following traumatic brain injury, Part II: attention and information processing speed. J Head Trauma Rehabil 2023; 38: 38–51.
- Sohlberg MM, Mateer CA. Effectiveness of an attention-training program. J Clin Exp Neuropsychol 1987; 9: 117–130.
- Barker-Collo SL, Feigin VL, Lawes CM, Parag V, Senior H, Rodgers A. Reducing attention deficits after stroke using attention process training: a randomized controlled trial. Stroke 2009; 40: 3292–3298.
- Cicerone KD, Goldin Y, Ganci K, Rosenbaum A, Wethe JV, Langenbahn DM, et al. Evidence-based cognitive rehabilitation: systematic review of the literature from 2009 through 2014. Arch Phys Med Rehabil 2019; 100: 1515–1533.
- Cicerone KD, Langenbahn DM, Braden C, Malec JF, Kalmar K, Fraas M, et al. Evidence-based cognitive rehabilitation: updated review of the literature from 2003 through 2008. Arch Phys Med Rehabil 2011; 92: 519–530.
- Engle JA, Kerns KA. Reinforcement learning in children with FASD. J Popul Ther Clin Pharmacol 2011; 18: e17–27.
- Cicerone KD, Dahlberg C, Kalmar K, Langenbahn DM, Malec JF, Bergquist TF, et al. Evidence-based cognitive rehabilitation: recommendations for clinical practice. Arch Phys Med Rehabil 2000; 81: 1596–1615.
- Markovic G, Bartfai A, Ekholm J, Nilsson C, Schult ML, Löfgren M. Daily management of attention dysfunction two-four years after brain injury and early cognitive rehabilitation with attention process training: a qualitative study. Neuropsychol Rehabil 2020; 30: 523–544.
- Pantoni L, Poggesi A, Diciotti S, Valenti R, Orsolini S, Della Rocca E, et al. Effect of attention training in mild cognitive impairment patients with subcortical vascular changes: The RehAtt Study. J Alzheimers Dis 2017; 60: 615–624.
- Govender P, Kalra L. Benefits of occupational therapy in stroke rehabilitation. Expert Rev Neurother 2007; 7: 1013–1019.
- Park HY, Maitra K, Martinez KM. The effect of occupation-based cognitive rehabilitation for traumatic brain injury: a meta-analysis of randomized controlled trials. Occup Ther Int 2015; 22: 104–116.
- Bartfai A, Markovic G, Sargenius Landahl K, Schult ML. The protocol and design of a randomised controlled study on training of attention within the first year after acquired brain injury. BMC Neurol 2014; 14: 102.
- Sargénius Landahl K. Evaluation of Attention training after acquired brain injury – an occupational perspective [rehabilitation medicine]. ProQuest Dissertations Publishing: Karolinska Institutet; 2021, Stockholm, Sweden.
- Sargénius Landahl K, Schult ML, Borg K, Bartfai A. Comparison of attention process training and activity-based attention training after acquired brain injury: a randomized controlled study. J Rehabil Med 2021; 53: jrm00235.
- Markovic G, Schult ML, Elg M, Bartfai A. Beneficial effects of early attention process training after acquired brain injury: a randomized controlled trial. J Rehabil Med 2020; 52: jrm00011.
- Bartfai A, Elg M, Schult ML, Markovic G. Predicting outcome for early attention training after acquired brain injury. Front Hum Neurosci 2022; 16: 767276.
- McCrea MA, Giacino JT, Barber J, Temkin NR, Nelson LD, Levin HS, et al. Functional outcomes over the first year after moderate to severe traumatic brain injury in the prospective, longitudinal TRACK-TBI study. JAMA Neurol 2021; 78: 982–992.
- Gilmore N, Katz DI, Kiran S. Acquired brain injury in adults: a review of pathophysiology, recovery, and rehabilitation. Perspect ASHA Spec Interest Groups 2021; 6: 714–727.
- Rabadi MH, Rabadi FM, Edelstein L, Peterson M. Cognitively impaired stroke patients do benefit from admission to an acute rehabilitation unit. Arch Phys Med Rehabil 2008; 89: 441–448.
- Fan MC, Li SF, Sun P, Bai GT, Wang N, Han C, et al. Early intensive rehabilitation for patients with traumatic brain injury: a prospective pilot trial. World Neurosurg 2020; 137: e183–e188.
- Hayden ME, Plenger P, Bison K, Kowalske K, Masel B, Qualls D. Treatment effect versus pretreatment recovery in persons with traumatic brain injury: a study regarding the effectiveness of postacute rehabilitation. PM & R 2013; 5: 319–327; quiz 27.
- León-Carrión J, Machuca-Murga F, Solís-Marcos I, León-Domínguez U, Domínguez-Morales Mdel R. The sooner patients begin neurorehabilitation, the better their functional outcome. Brain Inj 2013; 27: 1119–1123.
- Reid-Arndt SA, Schopp L, Brenneke L, Johnstone B, Poole AD. Evaluation of the traumatic brain injury early referral programme in Missouri. Brain Inj 2007; 21: 1295–1302.
- Saux G, Demey I, Rojas G, Feldberg C. Cognitive rehabilitation therapy after acquired brain injury in Argentina: psychosocial outcomes in connection with the time elapsed before treatment initiation. Brain Inj 2014; 28: 1447–1454.
- Schultz R, Tate RL, Perdices M. Neuropsychological recovery during the first 12 months after severe traumatic brain injury: a longitudinal study with monthly assessments. Neuropsychol Rehabil 2022;32: 1291–1323.
- Zarshenas S, Colantonio A, Horn SD, Jaglal S, Cullen N. Cognitive and motor recovery and predictors of long-term outcome in patients with traumatic brain injury. Arch Phys Med Rehabil 2019; 100: 1274–1282.
- Lo Buono V, Corallo F, Bramanti P, Marino S. Coping strategies and health-related quality of life after stroke. J Health Psychol 2017; 22: 16–28.
- Westerlind E, Persson HC, Törnbom K, Sunnerhagen KS. Return to work predicts perceived participation and autonomy by individuals with stroke. Disabil Rehabil 2020; 42: 3673–3678.
- Vestling M, Tufvesson B, Iwarsson S. Indicators for return to work after stroke and the importance of work for subjective well-being and life satisfaction. J Rehabil Med 2003; 35: 127–131.
- Donker-Cools BH, Daams JG, Wind H, Frings-Dresen MH. Effective return-to-work interventions after acquired brain injury: a systematic review. Brain Inj 2016; 30: 113–131.
- Gorgoraptis N, Zaw-Linn J, Feeney C, Tenorio-Jimenez C, Niemi M, Malik A, et al. Cognitive impairment and health-related quality of life following traumatic brain injury. NeuroRehabil 2019; 44: 321–331.
- Tanaka H, Toyonaga T, Hashimoto H. Functional and occupational characteristics predictive of a return to work within 18 months after stroke in Japan: implications for rehabilitation. Int Arch Occup Environ Health 2014; 87: 445–453.
- Dawson DR, Levine B, Schwartz ML, Stuss DT. Acute predictors of real-world outcomes following traumatic brain injury: a prospective study. Brain Inj 2004; 18: 221–238.
- Mateer CA, Sira CS. Cognitive and emotional consequences of TBI: intervention strategies for vocational rehabilitation. NeuroRehabil 2006; 21: 315–326.
- Vilkki JS, Juvela S, Siironen J, Ilvonen T, Varis J, Porras M. Relationship of local infarctions to cognitive and psychosocial impairments after aneurysmal subarachnoid hemorrhage. Neurosurg 2004; 55: 790–802; discussion –3.
- Willers C, Westerlind E, Borgström F, von Euler M, Sunnerhagen KS. Health insurance utilisation after ischaemic stroke in Sweden: a retrospective cohort study in a system of universal healthcare and social insurance. BMJ Open 2021; 11: e043826.
- Westerlind E, Persson HC, Eriksson M, Norrving B, Sunnerhagen KS. Return to work after stroke: a Swedish nationwide registry-based study. Acta Neurol Scand 2020; 141: 56–64.
- Tate RL, Simpson GK, McRae P. Return to work after trauamtic brain injury: a literature review. In: Escorpizo R, Brage S, Homa D, Stucki G, editors. Handbook of vocational rehabilitation and disability evaluation: application and implementation of the ICF 2015th edn. Springer International Publishing, Switzerland 2015, p. 275.
- Loetscher T, Potter KJ, Wong D, das Nair R. Cognitive rehabilitation for attention deficits following stroke. Cochrane Database Syst Rev 2019; 2019: CD002842.
- Rogers JM, Foord R, Stolwyk RJ, Wong D, Wilson PH. General and domain-specific effectiveness of cognitive remediation after stroke: systematic literature review and meta-analysis. Neuropsychol Rev 2018; 28: 285–309.
- Grönholm-Nyman P, Soveri A, Rinne JO, Ek E, Nyholm A, Stigsdotter Neely A, et al. Limited effects of set shifting training in healthy older adults. Front Aging Neurosci 2017; 9: 69.
- Zelinski EM. Far transfer in cognitive training of older adults. Restor Neurol Neurosci 2009; 27: 455–471.
- Markovic G, Schult ML, Bartfai A, Elg M. Statistical process control: a feasibility study of the application of time-series measurement in early neurorehabilitation after acquired brain injury. J Rehabil Med 2017; 49: 128–135.
- Markovic G, Schult ML, Bartfai A. The effect of sampling bias on generalizability in intervention trials after brain injury. Brain Inj 2017; 31: 9–15.
- Wiseman-Hakes C, MacDonald S, Keightley M. Perspectives on evidence based practice in ABI rehabilitation. “Relevant Research”: who decides? NeuroRehabil 2010; 26: 355–368.
- Balestroni G, Bertolotti G. [EuroQol-5D (EQ-5D): an instrument for measuring quality of life]. Monaldi Arch Chest Dis 2012; 78: 155–159.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997; 35: 1095–1108.
- Teni FS, Gerdtham UG, Leidl R, Henriksson M, Åström M, Sun S, et al. Inequality and heterogeneity in health-related quality of life: findings based on a large sample of cross-sectional EQ-5D-5L data from the Swedish general population. Qual Res Res 2022; 31: 697–712.
- Dorman PJ, Waddell F, Slattery J, Dennis M, Sandercock P. Is the EuroQol a valid measure of health-related quality of life after stroke? Stroke 1997; 28: 1876–1882.
- Eriksson G, Tham K, Kottorp A. A cross-diagnostic validation of an instrument measuring participation in everyday occupations: the Occupational Gaps Questionnaire (OGQ). Scand J Occup Ther 2013; 20: 152–160.
- Tuomi K, Ilmarinen J, Jahkola A, Katajarinne L, Tulkki A. Work Ability Index. Finnish Institute of Occupational Health, Helsinki, Finland; 1998.
- Lundin A, Leijon O, Vaez M, Hallgren M, Torgén M. Predictive validity of the Work Ability Index and its individual items in the general population. Scand J Public Health 2017; 45: 350–356.
- Pranjic N, Gonzales JMG, Cvejanov-Kezunović L. Perceived work ability index of public service employees in relation to ageing and gender: a comparison in three European countries. Zdr Varst 2019; 58: 179–188.
- Lundin A, Kjellberg K, Leijon O, Punnett L, Hemmingsson T. The association between self-assessed future work ability and long-term sickness absence, disability pension and unemployment in a general working population: a 7-year follow-up study. J Occup Rehabil 2016; 26: 195–203.
- Haskins ME, Cicerone KD, Dams-O’Connor K, Langenbahn DM, Shapiro-Rosenbaum A. Cognitive rehabilitation manual. Translating evidence-based recommendations into practice. Virginia, USA: American Congress of Rehabilitation Medicine, ACRM (BI-ISIG); 2013.
- Polatajko HJ, McEwen SE, Ryan JD, Baum CM. Pilot randomized controlled trial investigating cognitive strategy use to improve goal performance after stroke. Am J Occup Ther 2012; 66: 104–109.
- McEwen S, Polatajko H, Baum C, Rios J, Cirone D, Doherty M, et al. Combined cognitive-strategy and task-specific training improve transfer to untrained activities in subacute stroke: an exploratory randomized controlled trial. Neurorehabil Neural Repair 2015; 29: 526–536.
- Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale: L. Erlbaum Associates; 1988.
- Tanaka H, Toyonaga T, Hashimoto H. Functional and occupational characteristics associated with very early return to work after stroke in Japan. Arch Phys Med Rehabil 2011; 92: 743–748.
- Svensson JS, Westerlind E, Persson HC, Sunnerhagen KS. Occupational gaps 5 years after stroke. Brain Behav 2019; 9: 9e01234.
- Bergström AL, von Koch L, Andersson M, Tham K, Eriksson G. Participation in everyday life and life satisfaction in persons with stroke and their caregivers 3–6 months after onset. J Rehabil Med 2015; 47: 508–515.
- Bergström A, Guidetti S, Tham K, Eriksson G. Association between satisfaction and participation in everyday occupations after stroke. Scand J Occup Ther 2017; 24: 339–348.
- Lindgren I, Pessah-Rasmussen H, Gard G, Brogårdh C. Perceived work situation and work ability among persons who are working one year after stroke. J Rehabil Med 2022; 54: jrm00254.
- Hellman T, Bergström A, Eriksson G, Hansen Falkdal A, Johansson U. Return to work after stroke: important aspects shared and contrasted by five stakeholder groups. Work 2016; 55: 901–911.
- Scaratti C, Leonardi M, Sattin D, Schiavolin S, Willems M, Raggi A. Work-related difficulties in patients with traumatic brain injury: a systematic review on predictors and associated factors. Disabil Rehabil 2017; 39: 847–855.
- Watkin C, Phillips J, Radford K. What is a ‘return to work’ following traumatic brain injury? Analysis of work outcomes 12 months post TBI. Brain Inj 2020; 34: 68–77.
- van Velzen JM, van Bennekom CA, Edelaar MJ, Sluiter JK, Frings-Dresen MH. How many people return to work after acquired brain injury?: a systematic review. Brain Inj 2009; 23: 473–488.
- Wei X-J, Liu X-f, Fong KNK. Outcomes of return-to-work after stroke rehabilitation: a systematic review. Br J Occup Ther 2016; 79: 299–308.
- Donker-Cools B, Wind H, Frings-Dresen MHW. Prognostic factors of return to work after traumatic or non-traumatic acquired brain injury. Disabil Rehabil 2016; 38: 733–741.
- Saar K, Tolvanen A, Poutiainen E, Aro T. Returning to work after stroke: associations with cognitive performance, motivation, perceived working ability and barriers. J Rehabil Med 2023; 55: jrm00365.
- Edwards JD, Kapoor A, Linkewich E, Swartz RH. Return to work after young stroke: a systematic review. Int J Stroke 2018; 13: 243–256.
- La Torre G, Lia L, Francavilla F, Chiappetta M, De Sio S. Factors that facilitate and hinder the return to work after stroke: an overview of systematic reviews. Med Lav 2022; 113: e2022029.
- Sandqvist JL, Björk MA, Gullberg MT, Henriksson CM, Gerdle BU. Construct validity of the Assessment of Work Performance (AWP). Work 2009; 32: 211–218.
- Sargénius Landahl K, Sandqvist J, Bartfai A, Schult ML. Is a structured work task application for the assessment of work performance in a constructed environment, useful for patients with attention deficits? Diabil Rehabil 2021; 43: 1699–1709.
- Marom B, Jarus T, Josman N. The relationship between the Assessment of Motor and Process Skills (AMPS) and the Large Allen Cognitive Level (LACL) test in clients with stroke. Phys Occup Ther Geriat 2006; 24: 33–50.
- Sansonetti D, Hoffmann T. Cognitive assessment across the continuum of care: the importance of occupational performance-based assessment for individuals post-stroke and traumatic brain injury. Aust Occup Ther J 2013; 60: 334–342.
- Melby-Lervåg M, Hulme C. Is working memory training effective? A meta-analytic review. Dev Psychol 2013; 49: 270–291.
- Radomski MV, Anheluk M, Bartzen MP, Zola J. Effectiveness of interventions to address cognitive impairments and improve occupational performance after traumatic brain injury: a systematic review. Am J Occup Ther 2016; 70: 7003180050p1-9.
- Sigmundsdottir L, Longley WA, Tate RL. Computerised cognitive training in acquired brain injury: a systematic review of outcomes using the International Classification of Functioning (ICF). Neuropsychol Rehabil 2016; 26: 673–741.
- Eriksson G. Occupational Gaps Questionnaire (OGQ). Swedish Association of Occupational Therapists 2017, version 2.0, Nacka, Sweden (in Swedish).