ORIGINAL REPORT
DAILY PATTERNS OF FATIGUE AFTER SUBARACHNOID HAEMORRHAGE: AN ECOLOGICAL MOMENTARY ASSESSMENT STUDY
Elisabeth A. DE VRIES, MSC1,2, Majanka H. HEIJENBROK-KAL, PHD1,2, Fop VAN KOOTEN, PHD3, Marco GIURGIU, PHD4, Ulrich W. EBNER-PRIEMER, PHD4,5, Gerard M. RIBBERS, PHD1,2, Rita J. G. VAN DEN BERG-EMONS, PHD1 and Johannes B. J. BUSSMANN, PHD1
From the 1Department of Rehabilitation Medicine, Erasmus MC, University Medical Center Rotterdam, 2Rijndam Rehabilitation, Rotterdam, 3Department of Neurology, Erasmus MC, University Medical Center Rotterdam, Rotterdam, The Netherlands, 4Mental mHealth lab, Institute of Sports and Sports Science, Karlsruhe Institute of Technology, Karlsruhe, 5mHealth Methods in Psychiatry, Department of Psychiatry and Psychotherapy, Central Institute of Mental Health, Medical Faculty Mannheim, University of Heidelberg, Mannheim, Germany
Objective: To examine the daily course of, and factors associated with, momentary fatigue after subarachnoid haemorrhage, and to explore subgroups of patients with distinct diurnal patterns of fatigue.
Design: Observational study using ecological momentary assessment.
Subjects: A total of 41 participants with subarachnoid haemorrhage.
Methods: Patients with fatigue were included within one year post-onset. Momentary fatigue (scale 1–7) was assessed with repeated measurements (10–11 times/day) during 7 consecutive days. Multilevel-mixed-model analyses and latent-class trajectory modelling were conducted.
Results: Mean (standard deviation; SD) age of the group was 53.9 (13.0) years, 56% female, and mean (SD) time post-subarachnoid haemorrhage onset was 9.3 (3.2) months. Mean (SD) momentary fatigue over all days was 3.22 (1.47). Fatigue increased significantly (p < 0.001) over the day, and experiencing more burden of fatigue and day type (working day vs weekend day) were significantly (p < 0.05) associated with higher momentary fatigue. Three subgroups could be distinguished based on diurnal patterns of fatigue. The largest group (n = 17, 41.5%) showed an increasing daily pattern of fatigue.
Conclusion: Momentary fatigue in patients with subarachnoid haemorrhage increases over the day, and diurnal patterns of fatigue differ between participants. In addition to conventional measures, momentary measures of fatigue might provide valuable information for physicians to optimize personalized management of fatigue after subarachnoid haemorrhage.
LAY ABSTRACT
Fatigue is one of the most common symptoms after subarachnoid haemorrhage. Fatigue is usually assessed with a questionnaire, which gives a single score of fatigue over the preceding week(s). However, fatigue in people with subarachnoid haemorrhage may fluctuate over the day. Insight into these daily patterns of fatigue may help in developing adequate rehabilitation strategies to manage fatigue after subarachnoid haemorrhage. Therefore, this study examined the course of fatigue over the day. In addition, the study examined which factors are associated with daily fatigue and whether subgroups with different daily patterns of fatigue can be distinguished. It was found that fatigue increases over the day. In addition, patients who experienced more burden of fatigue in daily life had higher daily fatigue, and fatigue was higher on working days than on weekend days. Three subgroups with different daily patterns of fatigue could be distinguished. Most patients had an increasing pattern of fatigue.
Key words: subarachnoid haemorrhage; fatigue; stroke; ecological momentary assessment; latent class analysis.
Citation: J Rehabil Med 2023; 55: jrm6486. DOI: https://doi.org/10.2340/jrm.v55.6486
Copyright: © Published by Medical Journals Sweden, on behalf of the Foundation for Rehabilitation Information. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Aug 24, 2023; Published: Oct 18, 2023
Correspondence address: Elisabeth A. de Vries, Department of Rehabilitation Medicine, Erasmus MC, University Medical Center Rotterdam, Rotterdam, the Netherlands. E-mail: e.a.devries@erasmusmc.nl
Competing interests and funding: The authors have no conflicts of interest to declare.
Subarachnoid haemorrhage (SAH) is bleeding in the subarachnoid space of the brain, mostly caused by rupture of an aneurysm. SAH is a rather rare condition; it accounts for approximately 5% of all strokes (1) and the incidence rate is 9.1 per 100,000 people per year (2). The survival rate is approximately 65% (3); however, most SAH survivors have long-lasting impairments regarding fatigue, cognition and mood (4). These impairments highly affect daily functioning, especially considering the relatively young age of most people with SAH, which ranges between 40 and 60 years (1, 4). Fatigue is one of the most common and debilitating symptoms after SAH; the prevalence ranges between 31% and 90% (5, 6) and fatigue may even persist up to 10 years post SAH onset (7). Fatigue after SAH is found to be associated with impaired cognitive functioning, mood problems (4, 5, 8) and reduced participation and quality of life (7, 9, 10). In addition, most unmet needs of patients with SAH relate to fatigue (11). However, to date, intervention studies on management of fatigue in patients with neurological conditions show inconclusive results, which could be explained by a poor understanding of the aetiology of fatigue (12–15).
Fatigue can be quantified as a trait and/or a state symptom (16, 17). Trait fatigue can be considered a stable symptom and is often assessed retrospectively using questionnaires such as the Fatigue Severity Scale (FSS) or the Checklist Individual Strength (CIS). These questionnaires provide a mean fatigue score over the course of the previous week or weeks (17–20). State fatigue, hereafter referred to as momentary fatigue, reflects changes in fatigue during the day according to circumstances or events (17, 21). Momentary fatigue can be assessed using ecological momentary assessment (EMA), which is the method of choice to assess daily fluctuations and momentary states over a long period (22). EMA involves repetitive collection of real-time data on self-reported experiences of individuals in their natural environment (22). Through the use of smartphone applications, individuals can be prompted multiple times a day with 1 or more questions to assess how fatigued they feel at that specific moment in time. Notably, in patients with stroke and multiple sclerosis (MS) it has been found that momentary fatigue is only weakly associated with trait fatigue (FSS and CIS) (23, 24). This implies that increasing our understanding of momentary fatigue, may add to existing knowledge on trait fatigue, which may aid in developing effective strategies to reduce fatigue after SAH.
Several studies in patients with neurological illnesses have expanded insight into momentary fatigue by examining either the course of momentary fatigue over the day or factors associated with momentary fatigue (25). Momentary fatigue was found to increase during the day in patients with MS and this increase depended on sex and age (26). Another study showed that 4 groups of patients with MS could be distinguished based on their diurnal pattern of fatigue, with the 2 largest groups showing either an increasing profile or a stable high profile of fatigue over the day (24). Patients with traumatic brain injury who had higher variability in momentary fatigue reported more trait symptoms regarding depression, anxiety and affect than patients with less variability (27). In addition, in patients with MS momentary fatigue was higher in those reporting worse momentary mood. However, sleep quality, assessed on a daily basis, was not associated with momentary fatigue (28).
To our knowledge, to date, there are no studies of momentary fatigue after SAH. Gaining insight in the course of momentary fatigue over the day and potentially associated factors (e.g. sleep) may therefore increase our understanding of fatigue after SAH, which may aid in developing more specific and personalized strategies for the management of fatigue in this population. Therefore, the aims of the current study were, first, to examine the course of momentary fatigue over the day and to determine which factors are associated with momentary fatigue; and, secondly, to explore whether subgroups of patients with distinct diurnal patterns of fatigue can be distinguished. It was hypothesized that momentary fatigue increases over the day and is associated with sleep, mood and burden of fatigue.
METHODS
Participants and procedures
Consecutive patients diagnosed with SAH, who were treated at Erasmus MC University Medical Centre Rotterdam, the Netherlands, between August 2017 and May 2019 were screened for eligibility, and subsequently invited to participate in the study. In addition, patients who were treated at Elisabeth-TweeSteden Hospital between January 2019 and January 2020, were screened for eligibility, and invited to participate in the study.
The diagnosis of SAH was confirmed by computerized tomography (CT). In case CT was inconclusive, lumbar puncture was conducted to confirm SAH diagnosis. Patients were included if they were at least 18 years old, were between 3- and 12-months post-SAH onset, were living at home and were experiencing fatigue. This was checked by the researcher (EAV) in a telephone call before inclusion, by asking whether the patients had experienced fatigue since the SAH. Exclusion criteria were: previous stroke, having another chronic disease (including neurological diseases, e.g. cancer or MS), insufficient mastery of the Dutch language, or unable to understand verbal instructions and/or complete the questionnaires and e-diary. No formal sample size calculation has been performed for this study. Based on the number of participants used in previous comparable studies (23, 25, 28) and the high number of repeated measurements in the study design, a sample size of at least 40 participants was deemed sufficient. All patients signed an informed consent form before the start of the study. The study was approved by the medical ethics committee of Erasmus MC (MEC-2017-523).
Included patients were visited at home by a researcher. Before the home visit patients were asked to complete questionnaires, which were sent by post. During the home visit a semi-structured interview was conducted and the questionnaires were checked for completeness. In addition, patients received information about completing the electronic diary.
Ecological momentary assessment
EMA was used for 7 consecutive days with an electronic diary using the MovisensXS software (movisens GmbH, Karlsruhe, Germany) and mobile phone app (Version 1.3.4). Patients received a mobile phone (Alcatel U3) with the MovisensXS app installed on it that was running offline.
The primary outcome measure was momentary fatigue. Every day patients were prompted 10–11 times between 09.00 h and 21.00 h, with a single fatigue question (“How fatigued do you feel at this moment?”), which was answered on a 7-point Likert scale, ranging from 1 (not fatigued) to 7 (extremely fatigued). This item was used successfully in previous EMA studies (24, 26) and corresponds with the scale of fatigue questionnaires, such as the FSS (29). In addition, this fatigue question was found to have good convergent validity with Patient-Reported Outcomes Measurement Information System (PROMIS) fatigue items in a previous study (26). The time between 2 consecutive fatigue prompts within the 12-h time-frame was random, but was at least 45 min in order to enhance ecological validity. Patients were allowed to postpone prompts by 5, 10 or 15 min, or they could dismiss the prompt.
Secondarily, patients were prompted with questions to assess sleep duration, sleep quality and mood on a daily basis. If patients scored 1 of the fatigue questions a day as higher than 1, they were considered to have experienced fatigue that day. In that case, they received additional questions on their fatigue at 21.00 h. These extra questions addressed the type of fatigue, burden of fatigue, and ability to do activities despite their fatigue. All of these day level EMA questions are shown in Table I.
Patients’ characteristics
For descriptive purposes trait fatigue was assessed with the FSS (29). The FSS consists of 9 statements, scored on a 7-point Likert Scale, ranging from 1 (strongly disagree) to 7 (strongly agree). A cut-off score of ≥ 4 is used to distinguish fatigued from non-fatigued patients (29). The FSS is found reliable and valid to assess fatigue in patients with stroke (19).
Clinical characteristics including World Federation of Neurosurgical Societies (WFNS) classification, type of SAH (aneurysmatic (aneurysmal-SAH and non-aneurysmal-SAH) vs perimesencephalic, location of aneurysm (anterior vs posterior circulation), treatment modality aneurysm (endovascular (coiling and/or flowdiverter) vs neurosurgical (clipping)), serious SAH-related complications (re-bleed, hydrocephalus, ischaemia), history of hypertension (yes/no), length of hospital stay and discharge destination (home vs inpatient clinic (i.e. rehabilitation centre/nursing home)) were collected from the patient files. Several other characteristics were retrieved from the semi-structured interviews, including history of smoking, current smoking status, years of education, employment status (paid job vs no or unpaid job), and living status (alone vs with others).
Statistical analysis
Statistical analyses were performed using R (R Core Team, 2020, Vienna, Austria) and RStudio (RStudio Team, 2020, Boston, USA) Version 1.4.1717. Descriptive statistics were used to describe baseline characteristics of the study group and scores of the EMA questions.
To examine the course of fatigue over the day and potentially associated factors of momentary fatigue, multilevel-mixed-model analyses were conducted, with EMA fatigue as dependent variable (level-1) nested within participants (level-2). First, the course of momentary fatigue was examined by a model with momentary fatigue as dependent variable and time and time2 as predictors, and random intercepts and slopes for time. Subsequently, baseline characteristics, EMA outcomes and trait fatigue were added to separate univariable models with momentary fatigue as dependent variable, adjusted for time and time2. Finally, factors that were significantly (p < 0.05) associated with momentary fatigue in these models were added to the multivariable model with time and time2, adjusted for sex and age. The proportion of within-person variance and between-person variance that explained the total variance in fatigue (i.e. intraclass correlation (ICC)) was calculated by running an empty model. Instability in fatigue was expressed in the mean squared successive difference (MSSD) score (30).
Latent-class trajectory analysis was conducted using the LCMM package, to examine whether subgroups with distinct diurnal patterns of fatigue could be distinguished (31, 32). Data of multiple days are aggregated in this analysis. First the number of subgroups were selected based on the Bayesian Information Criterion (BIC) and subsequently the best model structure was determined. The model that best fitted the study data contained 3 subgroups, random intercepts and random slopes for time and time2, a common variance structure across subgroups and an unstructured variance-covariance matrix. Analysis of variance (ANOVA) tests were conducted to examine differences in patient characteristics between the 3 subgroups. Tukey post hoc tests were conducted to examine between which pairs of subgroups the difference(s) occurred. A significance level of p < 0.05 was used.
RESULTS
Study population
Of 98 eligible patients, 42 (43%) participated in the study. Reasons for not participating were not interested in participation (n = 19), being too fatigued (n = 3), not experiencing fatigue (n = 9) and other/unknown reason (n = 25). One patient was excluded from the analysis due to a response rate of less than 30% of EMA questions, resulting in a study population of 41 patients. Mean age was 53.9 years (SD 13.2). The majority of participants were female (56.1%) and mean time post SAH onset was 9.6 months (SD 2.04). Mean FSS score was 5.03 (SD 1.19, range 2.33–7.00) and 32 patients (78%) were fatigued (FSS ≥ 4). Sixty-two percent (n = 16) of patients who had a paid job still were still on sick leave at the time of the home-visit. Patients’ characteristics are shown in Table II.
EMA outcomes
Patients received a total of 3,039 fatigue questions, of which they completed 2,860 (179 missing and 5 invalid); a compliance rate of 94.1%. Mean momentary fatigue over the 7 days was 3.22 (SD 1.47), which was significantly (p < 0.05) lower than mean trait fatigue (5.03 SD 1.19). Instability (mean MSSD) in momentary fatigue was 0.85 (SD 0.80). Fig. 1 shows the momentary fatigue scores over the week of 4 random participants, starting on Monday and their mean FSS score. Mean scores of EMA outcomes over the week (i.e. fatigue, mood, burden of fatigue, sleep quality and sleep duration) are shown in Table III. All participants experienced fatigue on all days, except for 3 patients (7.3%), who had 1 day on which all fatigue questions were scored 1, indicating that they did not experience fatigue during that day. Mean momentary fatigue over the working days was 3.29 (SD 1.46) and over the weekend days 3.07 (SD 1.49). Thirty-three patients (81%) experienced general fatigue at least 1 day in the week, followed by physical fatigue (n = 25, 61%) and mental fatigue (n = 23, 56%). Only 7 patients (17%) experienced undefined fatigue at least 1 day in the week. Almost 60% of patients (n = 23) were not able to do everything they wanted to do due to their fatigue for the majority of the week (4 or more days).
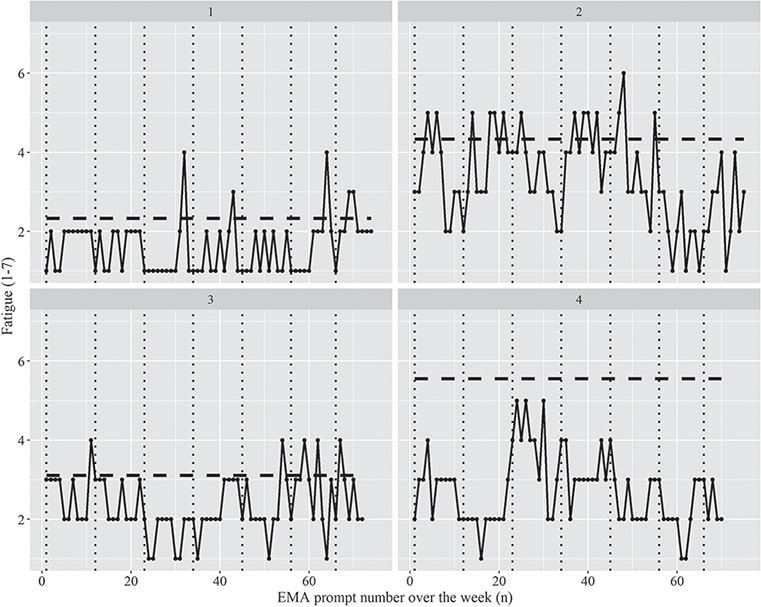
Fig. 1. Momentary Fatigue (black dots and black lines) over the week of 4 participants. Vertical black dotted lines represent the separation between the consecutive days, where the first day is Monday. The horizontal dashed lines represent the mean Fatigue Severity Scale (FSS) score (range 1–7), higher score means worse condition.
Course of fatigue and associated factors of momentary fatigue
On average, fatigue increased during the day and levelled off at the end of the day, given the significant (p < 0.001) effects for time (β = 0.220) and time2 (β = –0.010) in the model with only time effects as predictors.
Of all patient characteristics, only smoking status was associated with momentary fatigue in the univariable models. Smokers had significantly higher fatigue than non-smokers (β = 0.72, p < 0.05). Of the EMA outcomes, burden of fatigue and mood were associated with momentary fatigue, but sleep duration and sleep quality were not. Patients who perceived higher burden of fatigue (β = 0.72, p < 0.001) and worse mood (β = 0.72, p < 0.001) had significantly higher momentary fatigue. In addition, trait fatigue and day type were associated with momentary fatigue; patients who had higher scores on the FSS had significantly higher momentary fatigue (β = 0.27, p < 0.05) and momentary fatigue was significantly lower on weekend days than on working days (β = –0.20, p < 0.001).
The final multivariable model is shown below. A model was fitted with time, time2, day type (working vs weekend day), smoking status, FSS score, and burden of fatigue and mood scores over the week as predictors of interest, adjusted for sex and age and including random slopes for time. It was checked whether interactions of time with sex, type of day and smoking status, and random slopes for time2, significantly contributed to the model fit, and this was not the case.
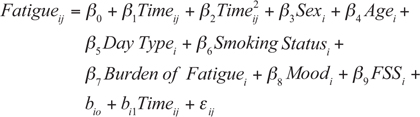
Results of the multilevel-mixed-model analysis are shown in Table IV. In addition to the time effects, more burden of fatigue (β7 = 0.680) was significantly associated with higher momentary fatigue. On working days momentary fatigue was significantly higher than on weekend days (β5 = –0.204). Sex, age, mood, smoking status and trait fatigue (FSS) were not associated with momentary fatigue (p > 0.05). Adding random slopes of time to the model significantly (p < 0.001) improved the model fit, indicating that diurnal patterns of fatigue varied among participants (Table SI). Within-subject variability accounted for 48% of the total variance in momentary fatigue (ICC = 0.522).
Subgroup analysis
Three subgroups could be distinguished based on diurnal patterns of fatigue (Fig. 2); a group with a stable low pattern (red line), a group with a slight increase in the morning and a subsequent decrease in the afternoon (green line), and a group with an increasing pattern (blue line). Most participants had an increasing pattern of fatigue (n = 17, 41.5%), followed by participants with a stable low pattern (n = 14, 34.1%) and with an increasing/decreasing pattern (n = 10, 24.4%). Characteristics of the subgroups are shown in Table V.
| Variables | Subgroup 1 (n = 14, 34%) | Subgroup 2 (n = 10, 24%) | Subgroup 3 (n = 17, 42%) |
| Age, mean (SD) | 50.4 (16.2) | 54.2 (9.43) | 56.6 (12.2) |
| Sex, female, n (%) | 10 (71.4) | 5 (50.0) | 8 (47.1) |
| Smoking, yes, n (%) | 1 (7.1) | 2 (20.0) | 6 (35.3) |
| Momentary fatigue, mean (SD) | 2.31 (0.69) | 2.88 (0.53) | 4.20 (0.75)* |
| Burden of fatigue, mean (SD) | 2.23 (0.88) | 2.82 (0.77) | 4.29 (0.95)* |
| Mood, mean (SD) | 1.33 (0.32) | 1.48 (0.63) | 1.99 (0.92)† |
| FSS score, mean (SD) | 4.13 (1.18) | 5.40 (0.71) | 5.55 (1.03)‡ |
| FSS ≥ 4, n (%) | 7 (50.0) | 10 (100.0) | 15 (88.2) |
| MSSD fatigue, mean (SD) | 0.71 (0.31) | 0.94 (0.58) | 0.90 (0.54) |
| *Subgroup 3 significantly higher than subgroup 1 and subgroup 2. †Subgroup 1 significantly lower than subgroup 3. ‡Subgroup 1 significantly lower than subgroup 2 and subgroup 3. | |||
| FSS: Fatigue Severity Scale; MSSD: Mean Squared Successive Difference; SD: standard deviation. | |||
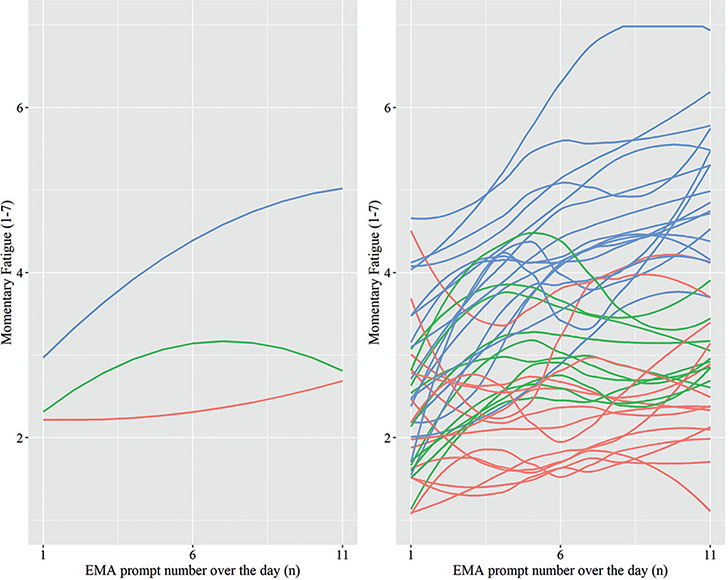
Fig. 2. Left panel diurnal patterns of fatigue of 3 subgroups; right panel diurnal patterns of participants per subgroup, subgroup 1 (red line); subgroup 2 (green line); subgroup 3 (blue line).
Significant differences were found between the subgroups for FSS score (F (2,38) = 8.38, p < 0.001), burden of fatigue (F (2,38) = 22.39, p < 0.001) and mood (F (2,38) = 3.86, p < 0.05), but not for age (F (2, 38) = 0.88) and instability in fatigue (MSSD, F (2,38) = 0.84). The stable low subgroup had significantly lower FSS scores than the increasing/decreasing subgroup (p < 0.05) and increasing subgroup (p < 0.01); the stable low and increasing/decreasing subgroups had significantly (p < 0.001) less burden of fatigue than the increasing subgroup; and the increasing subgroup had significantly worse mood than the stable low subgroup (p < 0.05).
DISCUSSION
Patients with SAH reported momentary fatigue scores that varied considerably over the day. Overall, momentary fatigue increased over the day and more burden of fatigue and type of day being a working vs weekend day were associated with higher momentary fatigue. In addition, it was found that diurnal patterns of fatigue differed between participants, so that 3 distinct subgroups could be distinguished.
As expected, on average, momentary fatigue increased over the day in patients with SAH. This is in line with findings from previous studies showed increasing fatigue over the day in both patients with MS (26, 28) and in healthy controls (28). Increasing fatigue during the day is often linked to activities and circumstances in daily life; however, patients with MS revealed a more rapid increase over the day in momentary fatigue than healthy controls who show highest fatigue at the end of the day (28). The increase in momentary fatigue over the day in patients with SAH found in the current study was comparable to that of patients with MS and, consequently, also more rapid than in healthy controls (28). Despite the difference in the aetiology of fatigue between people with SAH and MS, there are several papers that show similarities in their experience of chronic fatigue and its triggers and impact in daily life (16, 33, 34). Physical exertion was related to higher momentary fatigue in people with MS, but not in healthy controls (28) and in people with stroke it was found that momentary fatigue was higher after physical activity and during more effortful activities (35). Patients with SAH have worse physical fitness (36) than healthy controls, which is related to trait fatigue after SAH (37). Consequently, participating in activities of daily living after SAH may be more effortful, which may explain the more rapid accumulation of fatigue in patients with SAH compared with in healthy people.
Burden of fatigue and type of day were associated with momentary fatigue after SAH. Patients who experienced higher momentary fatigue also experienced more burden of fatigue in daily life. Higher trait fatigue is found to be associated with passive coping (38) and cognitive impairment (39) after SAH. It is possible that patients with SAH have difficulties coping with their fatigue in daily life, resulting in more perceived burden of fatigue. In addition, cognitive impairments after SAH might increase fatigue experience and consequently the burden of fatigue in daily life. The finding that patients perceived higher momentary fatigue on working days than on weekend days, may correspond with having to combine work duties with a role in family and social life during working days. In the current study, the majority of individuals with a paid job were still on sick leave. Additional explorative analyses revealed that the difference in fatigue between working days and weekend days was primarily caused by those who were still on sick leave. It is possible that this group has worse outcome, which may amplify the effect of having responsibilities regarding returning to work and family care on fatigue on working days. Mood, sleep quality and sleep duration and trait fatigue, were not associated with momentary fatigue. Regarding mood, these findings are in line with a previous study showing no associations between momentary fatigue and trait mood in patients with MS (40, 41). However, studies in patients with stroke and MS found an association between momentary mood and fatigue (28, 41). It was not possible to examine momentary associations in the current study, since mood was not assessed on the momentary level, but this would be of interest in future studies. People with SAH experience sleep disturbances (42), of which worse sleep latency and sleep quality were found to be associated with higher fatigue 2 months after SAH onset (43). In the current study, participants were assessed almost 10 months post-onset; they slept, a mean of 2 h longer every night and they had a higher sleep quality than those measured at 2 months post onset (43). This may explain why no association was found between sleep outcomes and momentary fatigue. The absence of a relationship between momentary fatigue and trait fatigue might be explained by burden of fatigue, which possibly accounted for the same variance in momentary fatigue as trait fatigue. Since burden of fatigue was assessed on a daily basis, it may be argued that it was a better predictor for momentary fatigue than trait fatigue, which was a mean score over the week.
Diurnal patterns of fatigue differed among participants and based on the patient-specific diurnal pattern of fatigue 3 subgroups could be distinguished. Strikingly, the largest group showed an increasing pattern of fatigue over the day, whereas fatigue was rather low in the other 2 groups. Based on the momentary fatigue pattern, the subgroup with the increasing pattern of fatigue clearly differed from the other 2 subgroups. However, trait fatigue (FSS) of the subgroups with the increasing pattern and the increasing/decreasing pattern, was similar. This implies that trait fatigue and momentary fatigue are different constructs; patients who have similar trait fatigue may have different diurnal patterns of fatigue, which is also found in patients after stroke (23). In addition, in the current study, mean trait fatigue was higher than mean momentary fatigue. This is in line with findings that people tend to overestimate their symptoms on retrospective questionnaires, since more salient symptoms are remembered better than no symptoms (22). In addition, cognitive impairments may induce recall bias on retrospective questionnaires (44), which may have caused differences in momentary fatigue and trait fatigue outcomes found in the current study. Due to the small number of people per subgroup, it was not possible to examine the role of potential confounding variables related to the clinical characteristics of the participants. However, based on studies on trait fatigue after SAH it can be suggested that characteristics including more severe SAH, complications such as vasospasm, re-bleed or delayed cerebral ischaemia, and smoking are predictive of being in the subgroup with the increasing pattern of fatigue (45–47). It would be of interest to examine not only the role of these confounding clinical variables in future studies, but also to examine whether being in the subgroup with the increasing pattern of fatigue relates to worse participation in daily life and health-related quality of life.
Patients with SAH reported momentary fatigue scores that varied considerably during the day. This is in line with previous studies in patients with traumatic brain injury (27) and MS (26, 28). The results of this study imply that by just assessing trait fatigue with conventional retrospective questionnaires, aspects of fatigue as experienced by patients after SAH in daily life might be missed. EMA measures of fatigue may provide valuable information for physicians to establish personalized programmes to manage fatigue after SAH. The results of the current study may be valuable for psychoeducation in rehabilitation and to guide patients in planning their activities, thereby preventing fatigue from accumulating over the day. In addition, the current results may have consequences for the timing of assessing fatigue (i.e. always at the same time of the day) and rehabilitation sessions. Finally, identifying patients with an increasing pattern of fatigue is important, because, for this group, attention should be paid to mood problems and high burden of fatigue. Higher momentary fatigue may be preceded by effortful activities and also may affect subsequent behaviour (48). Future studies should examine circumstances or situations (e.g. poor work-life balance, physical activity) in which patients perceive high levels of fatigue, which can subsequently be targeted in rehabilitation aimed at reducing or coping with fatigue.
One of the strengths of this study was assessing fatigue via EMA, thereby enabling examination of real-time daily fluctuations and preventing recall bias, which is particularly important in patients with cognitive impairments. Multiple days of assessment enabled robust estimates of within-days trajectories, and the compliance rate was excellent, indicating that it is feasible to use EMA over a week in patients with SAH.
Several limitations of this study should be considered. First, we did not assess other constructs, such as mood, type of fatigue or burden of fatigue multiple times a day. Therefore, momentary associations between these constructs and fatigue could not be analysed. Considering EMA feasibility, we focused on frequently described factors known to affect fatigue after SAH, even though other factors, such as cognition, company, and medication use, could also potentially affect momentary fatigue. Another limitation is the sample size. However, given the many repeated measurements within persons, the large number of observations was appropriate for multilevel analyses. Although subgroup analysis resulted in lower numbers of people per group, we could show significant group differences. Also the representativeness of our group may be debated, since less than 50% of eligible patients participated in the study. However, baseline characteristics, including age, sex and WFNS grade, were similar to those reported in other studies in people with SAH conducted in the Netherlands (39, 49, 50), which supports representativeness. As assessing momentary fatigue in daily life is still an innovative approach, norm values and extensively validated variables are missing. Likewise, divergent validity of momentary fatigue and burden of fatigue deserves further research.
In conclusion, momentary fatigue after SAH is not stable, but increases over the day, which may be associated with experienced burden of fatigue and type of day. Three distinct diurnal patterns of fatigue can be found in subgroups of patients with SAH. Assessing momentary fatigue provides insight into real-time daily patterns of fatigue, which are missed in conventional assessment of trait fatigue. Measures of momentary fatigue can therefore add to existing knowledge on fatigue and may inform rehabilitation professionals to develop or optimize personalized programmes for the management of fatigue after SAH.
ACKNOWLEDGEMENTS
This research was funded by the Erasmus MC Efficiency Research Pilot Grant: 2018-18106.
REFERENCES
- Macdonald RL, Schweizer TA. Spontaneous subarachnoid haemorrhage. Lancet 2017; 389: 655–666.
- de Rooij NK, Linn FH, van der Plas JA, Algra A, Rinkel GJ. Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatry 2007; 78: 1365–1372.
- Rinkel GJ, Algra A. Long-term outcomes of patients with aneurysmal subarachnoid haemorrhage. Lancet Neurol 2011; 10: 349–356.
- Al-Khindi T, Macdonald RL, Schweizer TA. Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke 2010; 41: 519–536.
- Kutlubaev MA, Barugh AJ, Mead GE. Fatigue after subarachnoid haemorrhage: a systematic review. J Psychosom Res 2012; 72: 305–310.
- Alghamdi I, Ariti C, Williams A, Wood E, Hewitt J. Prevalence of fatigue after stroke: a systematic review and meta-analysis. Eur Stroke J 2021; 6: 319–332.
- Buunk AM, Groen RJ, Veenstra WS, Spikman JM. Leisure and social participation in patients 4–10 years after aneurysmal subarachnoid haemorrhage. Brain Inj 2015; 29: 1589–1596.
- Boerboom W, van Zandvoort MJ, van Kooten F, Khajeh L, Visser-Meily JM, Ribbers GM, et al. Long-term fatigue after perimesencephalic subarachnoid haemorrhage in relation to cognitive functioning, mood and comorbidity. Disabil Rehabil 2017; 39: 928–933.
- de Vries EA, Boerboom W, van den Berg-Emons R, van Kooten F, Ribbers GM, Heijenbrok-Kal MH. Fatigue in relation to long-term participation outcome in aneurysmal subarachnoid haemorrhage survivors. J Rehabil Med 2021; 53: jrm00173.
- Powell J, Kitchen N, Heslin J, Greenwood R. Psychosocial outcomes at 18 months after good neurological recovery from aneurysmal subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry 2004; 75: 1119–1124.
- Dulhanty LH, Hulme S, Vail A, Patel HC, Tyson SF. The self-reported needs of patients following subarachnoid hemorrhage (SAH). Disabil Rehabil 2019; 42: 3450–3456.
- Su Y, Yuki M, Otsuki M. Non-pharmacological interventions for post-stroke fatigue: systematic review and network meta-analysis. J Clin Med 2020; 9: 621.
- Wu S, Kutlubaev MA, Chun HY, Cowey E, Pollock A, Macleod MR, et al. Interventions for post-stroke fatigue. Cochrane Database Syst Rev 2015;2015: CD007030.
- Elbers RG, Verhoef J, van Wegen EE, Berendse HW, Kwakkel G. Interventions for fatigue in Parkinson’s disease. Cochrane Database Syst Rev 2015; 2015: CD010925.
- Ali A, Morfin J, Mills J, Pasipanodya EC, Maas YJ, Huang E, et al. Fatigue after traumatic brain injury: a systematic review. J Head Trauma Rehabil 2022; 37: 249–257.
- Kluger BM, Krupp LB, Enoka RM. Fatigue and fatigability in neurologic illnesses: proposal for a unified taxonomy. Neurology 2013; 80: 409–416.
- Enoka RM, Almuklass AM, Alenazy M, Alvarez E, Duchateau J. Distinguishing between fatigue and fatigability in multiple sclerosis. Neurorehabil Neural Repair 2021; 35: 960–973.
- Dittner AJ, Wessely SC, Brown RG. The assessment of fatigue: a practical guide for clinicians and researchers. J Psychosom Res 2004; 56: 157–170.
- Nadarajah M, Mazlan M, Abdul-Latif L, Goh HT. Test-retest reliability, internal consistency and concurrent validity of Fatigue Severity Scale in measuring post-stroke fatigue. Eur J Phys Rehabil Med 2016.
- De Doncker W, Dantzer R, Ormstad H, Kuppuswamy A. Mechanisms of poststroke fatigue. J Neurol Neurosurg Psychiatry 2018; 89: 287–293.
- Enoka RM, Duchateau J. Translating fatigue to human performance. Med Sci Sports Exerc 2016; 48: 2228–2238.
- Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol 2008; 4: 1–32.
- Lenaert B, van Kampen N, van Heugten C, Ponds R. Real-time measurement of post-stroke fatigue in daily life and its relationship with the retrospective Fatigue Severity Scale. Neuropsychol Rehabil 2020; 32: 1–15.
- Heine M, van den Akker LE, Blikman L, Hoekstra T, van Munster E, Verschuren O, et al. Real-time assessment of fatigue in patients with multiple sclerosis: how does it relate to commonly used self-report fatigue questionnaires? Arch Phys Med Rehabil 2016; 97: 1887–1894.
- Juengst SB, Terhorst L, Nabasny A, Wallace T, Weaver JA, Osborne CL, et al. Use of mHealth technology for patient-reported outcomes in community-dwelling adults with acquired brain injuries: a scoping review. Int J Environ Res Public Health 2021; 18: 2173.
- Kratz AL, Murphy SL, Braley TJ. Ecological momentary assessment of pain, fatigue, depressive, and cognitive symptoms reveals significant daily variability in multiple sclerosis. Arch Phys Med Rehabil 2017; 98: 2142–2150.
- Juengst SB, Terhorst L, Kew CL, Wagner AK. Variability in daily self-reported emotional symptoms and fatigue measured over eight weeks in community dwelling individuals with traumatic brain injury. Brain Inj 2019; 33: 567–573.
- Powell DJH, Liossi C, Schlotz W, Moss-Morris R. Tracking daily fatigue fluctuations in multiple sclerosis: ecological momentary assessment provides unique insights. J Behav Med 2017; 40: 772–783.
- Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 1989; 46: 1121–1123.
- Ebner-Priemer UW, Eid M, Kleindienst N, Stabenow S, Trull TJ. Analytic strategies for understanding affective (in)stability and other dynamic processes in psychopathology. J Abnorm Psychol 2009; 118: 195–202.
- Proust-Lima C, Philipps V, Liquet B. Estimation of extended mixed models using latent classes and latent processes: the R Package lcmm. J Stat Software 2017; 78: 1–56.
- Lennon H, Kelly S, Sperrin M, Buchan I, Cross AJ, Leitzmann M, et al. Framework to construct and interpret latent class trajectory modelling. BMJ Open 2018; 8: e020683.
- Chaudhuri A, Behan PO. Fatigue in neurological disorders. Lancet 2004; 363: 978–988.
- Penner IK, Paul F. Fatigue as a symptom or comorbidity of neurological diseases. Nat Rev Neurol 2017; 13: 662–675.
- Lenaert B, Neijmeijer M, van Kampen N, van Heugten C, Ponds R. Poststroke fatigue and daily activity patterns during outpatient rehabilitation: an experience sampling method study. Arch Phys Med Rehabil 2020; 101: 1001–1008.
- Harmsen WJ, Ribbers GM, Zegers B, Sneekes EM, Heijenbrok-Kal MH, Khajeh L, et al. Impaired cardiorespiratory fitness after aneurysmal subarachnoid hemorrhage. J Rehabil Med 2016; 48: 769–775.
- Harmsen WJ, Ribbers GM, Heijenbrok-Kal MH, Khajeh L, Sneekes EM, van Kooten F, et al. Fatigue after aneurysmal subarachnoid hemorrhage is highly prevalent in the first-year postonset and related to low physical fitness: a longitudinal study. Am J Phys Med Rehabil 2019; 98: 7–13.
- Passier PE, Post MW, van Zandvoort MJ, Rinkel GJ, Lindeman E, Visser-Meily JM. Predicting fatigue 1 year after aneurysmal subarachnoid hemorrhage. J Neurol 2011; 258: 1091–1097.
- Boerboom W, Heijenbrok-Kal MH, Khajeh L, van Kooten F, Ribbers GM. Differences in cognitive and emotional outcomes between patients with perimesencephalic and aneurysmal subarachnoid haemorrhage. J Rehabil Med 2014; 46: 28–32.
- Kratz AL, Murphy SL, Braley TJ. Pain, fatigue, and cognitive symptoms are temporally associated within but not across days in multiple sclerosis. Arch Phys Med Rehabil 2017; 98: 2151–2159.
- Lau SCL, Connor LT, Skidmore ER, King AA, Lee JM, Baum CM. The moderating role of motivation in the real-time associations of fatigue, cognitive complaints, and pain with depressed mood among stroke survivors: an ecological momentary assessment study. Arch Phys Med Rehabil 2023; 104: 761–768.
- Schuiling WJ, Rinkel GJ, Walchenbach R, de Weerd AW. Disorders of sleep and wake in patients after subarachnoid hemorrhage. Stroke 2005; 36: 578–582.
- Byun E, McCurry SM, Opp M, Liu D, Becker KJ, Thompson HJ. Self-efficacy is associated with better sleep quality and sleep efficiency in adults with subarachnoid hemorrhage. J Clin Neurosci 2020; 73: 173–178.
- Forster SD, Gauggel S, Petershofer A, Völzke V, Mainz V. Ecological momentary assessment in patients with an acquired brain injury: a pilot study on compliance and fluctuations. Front Neurol 2020; 11: 115.
- Claassen J, Park S. Spontaneous subarachnoid haemorrhage. Lancet 2022; 400: 846–862.
- Western E, Sorteberg A, Brunborg C, Nordenmark TH. Prevalence and predictors of fatigue after aneurysmal subarachnoid hemorrhage. Acta Neurochir 2020; 162: 3107–3116.
- Huenges Wajer IMC, Hendriks ME, Witkamp TD, Hendrikse J, Rinkel GJE, Visser-Meily JMA, et al. The relationship between ischaemic brain lesions and cognitive outcome after aneurysmal subarachnoid haemorrhage. J Neurol 2019; 266: 2252–2257.
- Band R, Barrowclough C, Caldwell K, Emsley R, Wearden A. Activity patterns in response to symptoms in patients being treated for chronic fatigue syndrome: an experience sampling methodology study. Health Psychol 2017; 36: 264–269.
- Kruisheer EM, Huenges Wajer IMC, Visser-Meily JMA, Post MWM. Course of participation after subarachnoid hemorrhage. J Stroke Cerebrovasc Dis 2017; 26: 1000–1006.
- Buunk AM, Spikman JM, Metzemaekers JDM, van Dijk JMC, Groen RJM. Return to work after subarachnoid hemorrhage: the influence of cognitive deficits. PloS One 2019; 14 e0220972.