ORIGINAL REPORT
COMPARING THE EFFECT OF IMPLANTED PERONEAL NERVE STIMULATION AND ANKLE-FOOT ORTHOSIS ON GAIT KINEMATICS IN CHRONIC HEMIPARESIS: A RANDOMIZED CONTROLLED TRIAL
Emilie HUTIN, PhD1–3, Mouna GHÉDIRA, PT, PhD1,2, Maria VINTI, PT, PhD1–3, Sanaa TAZI, MD1,2, Jean-MICHEL GRACIES, MD, PhD1,3 and Philippe DECQ, MD, PhD1–3
From the 1Laboratoire Analyse et Restauration du Mouvement, Service de Rééducation Neurolocomotrice, Hôpitaux Universitaires Henri Mondor, AP-HP, Créteil, France, 2Service de Neurochirurgie, Hôpitaux Universitaires Henri Mondor, AP-HP, Créteil, France and 3Institut de Biomécanique Humaine Georges Charpak, Arts et Métiers ParisTech, Université Paris XIII, Paris, France
Objective: Impaired ankle dorsiflexion in hemiparesis may be treated with ankle-foot orthosis or functional electrical stimulation. Semi-implanted selective functional electrical stimulation uses independent stimulations of deep and superficial peroneal nerves. The aim of this study was to compare gait kinematics using ankle-foot orthosis or semi-implanted selective functional electrical stimulation over 6 months in hemiparesis.
Methods: Subjects with chronic hemiparesis, randomized into ankle-foot orthosis or semi-implanted selective functional electrical stimulation groups, underwent comfortable gait analysis without and with device OFF and ON, before, and 3 and 6 months after treatment onset. The effects of condition, visit and group on gait kinematics (analysis of variance; ANOVA) were analysed.
Results: A total of 27 subjects were included (ankle-foot orthosis, n = 13; semi-implanted selective functional electrical stimulation, n = 14). The only between-group difference in changes from OFF to ON conditions was a deteriorated ankle dorsiflexion speed with ankle-foot orthosis at month 6 (condition*group, p = 0.04; ankle-foot orthosis, –60%, p = 0.02; semi-implanted selective functional electrical stimulation, non significant). Both groups pooled, from OFF to ON gait speed (+ 0.07 m/s; + 10%), cadence (+ 4%), step length (+ 6%) and peak ankle dorsiflexion (+ 6°) increased, and peak ankle inversion (–5°) and peak knee flexion (–2°) decreased (p < 0.001); finally, peak knee flexion in the OFF condition increased (+ 2°, p = 0.03).
Conclusion: Semi-implanted selective functional electrical stimulation and ankle-foot orthosis similarly impacted gait kinematics in chronic hemiparesis after 6 months of use. Ankle dorsiflexion speed in swing deteriorated markedly with ankle-foot orthosis.
LAY ABSTRACT
After a central nervous system injury, walking disorders are associated with ankle dorsiflexion and foot eversion in the paretic limb during the swing phase. Movement of the ankle can be partially corrected with ankle-foot orthosis (AFO) or functional electrical stimulation (FES). The semi-implanted selective FES (SIS-FES) is an advanced FES device using independent stimulations of deep and superficial peroneal nerves, to separately control movements of ankle dorsiflexion, hallucis extension and foot eversion, and to optimize FES-associated walking improvements. This study compared walking using AFO or SIS-FES over 6 months in hemiparesis. A total of 27 patients with chronic hemiparesis, randomized into AFO or SIS-FES groups, underwent comfortable walking analysis without and with device OFF and ON, before, and 3 and 6 months after treatment onset. SIS-FES and AFO similarly improved walking speed, cadence, step length, ankle dorsiflexion and foot eversion, while ankle dorsiflexion speed in swing markedly deteriorated with AFO.
Key words: stroke; hemiparesis; assistive device; functional electrical stimulation; motion analysis.
Citation: J Rehabil Med 2023; 55: jrm7130. DOI: https://doi.org/10.2340/jrm.v55.7130.
Copyright: © Published by Medical Journals Sweden, on behalf of the Foundation for Rehabilitation Information. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: May 31, 2023; Published: Aug 7, 2023
Correspondence address: Emilie Hutin, Laboratory Analysis and Restoration of Movement, Department of Physical Medicine and Rehabilitation, Henri Mondor Hospital, 51, avenue du Maréchal de Lattre de Tassigny, FR-94000 Créteil, France. E-mail: emilie.hutin@aphp.fr
Competing interests and funding: The authors have no conflicts of interest to declare.
Foot clearance during the swing phase of gait is commonly impaired in hemiparesis due to central nervous system lesions. Three factors are commonly involved: dorsiflexor paresis, and plantar flexor shortening and plantar flexor overactivity (1, 2). A classic approach has been to passively keep the ankle up while walking, using ankle-foot orthoses (AFO), usually made of thermoplastic material (3). AFOs, which aim to keep the ankle close to zero degrees of dorsiflexion, may facilitate foot clearance in swing as well as heel initial contact; they may also prevent ankle sprain in case of ankle inversion. AFO use increases walking speed (4, 5), and improves knee kinematics during the stance phase (6). Another approach is to provide “active” assistance, by electrically stimulating the peroneal nerve during swing, a concept known as functional electrical stimulation (FES), which was developed by Liberson et al. in 1961 (7–9). Randomized controlled trials with at least 10 participants in each group to compare the effects of FES vs AFO (10–16), and of FES vs conventional physical therapy (17–19), have provided controversial results on walking speed (Table I). However, most studies have found overall no superiority of FES compared with control interventions as far as walking speed is concerned (20, 21). With most prototypes, placement of the electrodes over the skin to achieve optimal effects has been a source of variability in effects, which has limited the practicality of classic FES (22, 23), although this may be mitigated to some extent by mounting electrodes on a cuff worn around the leg.
| Stimulator (channels, electrodes, sensors) | Study design, groups (n) | Walking distance, speed | Intervention duration | Effect on speed (change from baseline) | |||
| Orthotic | Training | Therapeutic | Combined | ||||
| Odstock (1, surface, foot-switch) (17) | RCT, FES/PT (16/16) | 3×10 m, fast | 4 weeks | FES, ns | FES, ns; PT, ns | FES, ns; PT, ns | FES, ns |
| 12 weeks | FES, ns | FES, ns; PT, ns | FES, ns; PT, ns | FES, +20%* | |||
| Odstock (1, surface, foot-switch) (10) | ROWS, FES/AFO (14) | 5 m, comf. | 1 session | FES, +12%* | |||
| AFO, +24%* | |||||||
| Intergroup, ns | |||||||
| STIMuSTEP (2, implanted, foot-switch) (11) | RCT, FES/AFO (14/13) | 4×10 m, comf. | 4, 8, 12, 26 weeks | FES, ns; AFO, ns | FES, ns; AFO, ns | ||
| Over each period | FES, ↗*; AFO, ns | FES, ns; AFO, ns | |||||
| FES > AFO* | |||||||
| EMS (1, surface, foot-switch) (18) | RCT, FES+PT/PT (16/14) | 10 m, comf. | 12 weeks | FES+PT, +26%*** | |||
| PT, +12%*** | |||||||
| Intergroup, ns | |||||||
| WalkAide (1, surface, tilt sensor) (12) | RCT, FES/AFO (10/10) | 10 m, comf. | 4 weeks | FES, +61%** | |||
| AFO, +29%* | |||||||
| Intergroup* | |||||||
| WalkAide (1, surface, tilt sensor) (13) | mRCOT, FES-AFO/ AFO-FES/AFO-AFO (36/29/21) | 10 m, comf. | 6 weeks | FES, +11%* | FES, +26%* | FES, +37%* | |
| AFO, +19%* | AFO, +18%* | AFO, +37%* | |||||
| Intergroup, ns | Intergroup, ns | Intergroup, ns | |||||
| NESS L300 (1, surface, foot-switch) (14) | RCT, FES/AFO (99/95) | 10 m, comf. | 30 weeks | FES, +0.09 m/s* | FES, +0.06 m/s* | FES, +0.09 m/s* | FES, +0.15 m/s* |
| AFO, +0.07 m/s* | AFO, +0.08 m/s* | AFO, +0.10 m/s* | AFO, +0.14 m/s* | ||||
| Intergroup, ns | Intergroup, ns | Intergroup, ns | Intergroup, ns | ||||
| 10 m, fast | 30 weeks | FES, +0.09 m/s* | FES, +0.07 m/s* | FES, +0.05 m/s* | FES, +0.17 m/s* | ||
| AFO, +0.05 m/s* | AFO, +0.08 m/s* | AFO, +0.06 m/s* | AFO, +0.13 m/s* | ||||
| FES > AFO* | Intergroup, ns | Intergroup, ns | Intergroup, ns | ||||
| WalkAide (1, surface, tilt sensor) (15) | mRCT, FES/AFO (187/221) | 10 m, comf. | 6 months | FES, +41%*** | |||
| AFO, +40%*** | |||||||
| Intergroup, ns | |||||||
| WalkAide (1, surface, tilt sensor) (16) | mRCT, FES/AFO (180/204) | 10 m, comf. | 12 months | FES, +44%*** | |||
| AFO, +35%*** | |||||||
| Intergroup, ns | |||||||
| WalkAide (1, surface, tilt sensor) (19) | RCT, FES/PT (10/10) | 8 cycles, comf. | 10 weeks | FES, ns; PT, ns | |||
| AFO: ankle-foot orthosis; PT: physical therapy; RCT: randomized controlled trial; mRCT: multicentric randomized controlled trial; ROWS: random order within subject; RCOT: randomized crossover trial; mRCOT: multicentric randomized crossover trial; comf.: comfortable; ns: non-significant. Intra- and inter-group comparisons, ***p<0.001; **p<0.01; *p<0.05. | |||||||
An advanced FES device, designed with dual stimulation implanted around the peroneal nerve, has been developed as a practical alternative to overcome the difficulty of placing electrodes and potential skin irritation (11, 23–26). This semi-implanted selective FES (SIS-FES) allows the intensity of stimulations to be individually adjusted to the deep branch, activating the tibialis anterior, peroneus tertius and extensor hallucis longus, and to the superficial branch, activating the peroneus longus and peroneus brevis. Movements of ankle dorsiflexion, hallucis extension and foot eversion can thus be controlled separately in order to optimize FES-associated walking improvements (11, 23–26). A single randomized controlled trial comparing SIS-FES with AFO over 26 weeks of use showed higher walking speed improvement with the SIS-FES ON (11), and higher voluntary recruitment of tibialis anterior with the device OFF (22).
In terms of pathophysiology and effects of SIS-FES on the kinematics of the paretic lower limb, data is still scarce, particularly in the swing phase (27, 28). Among the 3 main factors of foot clearance impairment (1, 2), AFOs could be considered to address plantar flexor shortening and spastic dystonia, whereas the SIS-FES might primarily target dorsiflexor paresis. Recent studies showed dorsiflexor paresis and plantar flexor co-contraction as key factors in walking impairment, ahead of plantar flexor spasticity (29, 30). The SIS-FES might then provide interesting impact on ankle kinematics during gait. The objective of the current study in chronic hemiparesis was to compare various types of clinical and kinematic effects of AFO and SIS-FES after 3 and 6 months of use: orthotic (changes from OFF to ON the device), training (performances ON after a period of use), therapeutic (changes OFF the device after a period of use) and combined effects. The main hypothesis of the study was that SIS-FES might increase comfortable walking speed more than AFO (different orthotic effects), through quantitative increase in agonist dorsiflexor activity during the swing phase.
METHODS
Study context and ethics
This is the primary report of a multicentre randomized controlled trial, STEPSTIM (ClinicalTrials.gov Identifier: NCT01415700; P070155; ID-RCB number 2007-A01444-49), which aimed to compare the effects of SIS-FES and AFO in individuals with chronic hemiparesis, specifically focusing on three-dimensionnal quantitative gait analysis using a computer-aided motion analysis and force measurement system. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines, and was approved by the relevant research ethics committee. The primary outcome was the difference in change in comfortable walking speed with shoes, calculated from 3-dimensional gait analysis over 8 m from OFF to ON between the 2 tested devices after 3 months of device use. The secondary outcomes involved a number of gait kinematic parameters, as described in Assessments, below. Study interventions and assessments were carried out in the Department of Neurosurgery and in an Analysis and Restoration of Movement Laboratory. Four recruiting centres were involved during the 3-year study period. All subjects provided written informed consent to the inclusion of material pertaining to themselves, acknowledging that they could not be identified through any publication from the STEPSTIM trial. All mandatory laboratory health and safety procedures were complied with in the course of conducting the experimental work (31).
Participants
Inclusion criteria were: age ≥ 18 years, central nervous lesion that occurred at least 12 months before enrolment; ability to walk over a distance of 50 m with or without assistive device; impaired paretic ankle dorsiflexion during the swing phase of gait as per the investigator; ability to walk with an AFO; possibility to stimulate the peroneal nerve; absence of neurotomy in the paretic lower limb in the past 12 months before enrolment; absence of neurolytic alcohol or phenol block in the past 6 months; absence of botulinum injection in the paretic lower limb in the past 4 months; written consent for participation. Non-inclusion criteria were: maximal passive ankle dorsiflexion in the paretic limb with knee extended < 0° (i.e. Tardieu XV1 gastrocnemius < 90°); use of orthopaedic shoes covering the malleolus; any contraindication to general anaesthesia; ongoing use of another implanted stimulator (included cardiac pacemaker); systemic use of synaptic depressors, such as neuroleptics, gamma-amino-butyric ascid (GABA)-ergic agents, antidepressants or any other drug that might affect walking ability; untreated epilepsy; peripheral nervous system lesion; pregnant or nursing woman; absence of affiliation to healthcare coverage.
Intervention
Participants were randomized into the AFO or the SIS-FES group by an independent research unit. Five visits to set up the intervention were planned, day 1 (D1) being defined as the day of first use of the device: 2 months before D1 (M–2), 30 days before D1 (D–30, only in the SIS-FES group), 15 days before D1 (D–15), D1 and 15 days after D1 (D15; Fig. 1). After D1, participants were asked to use the device in everyday life as much as possible, both in the home and outdoors. For both groups, it was requested that ongoing rehabilitation care at inclusion be left unchanged during the study.
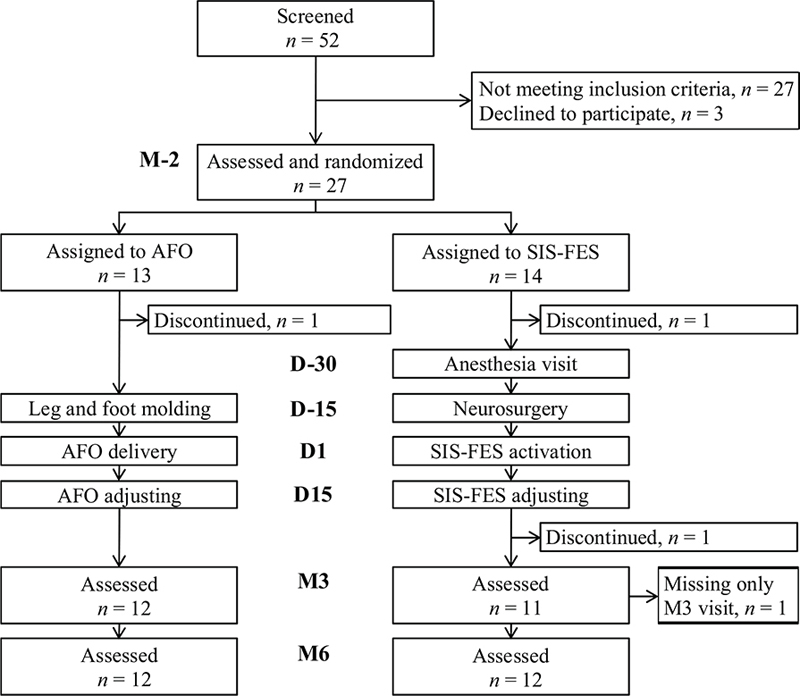
Fig. 1. Flowchart of patients through the study. The participants, randomized into ankle-foot orthosis (AFO) or semi-implanted selective functional electrical stimulation (SIS-FES) groups, underwent gait assessment in the 3D laboratory 2 months before (M–2), and 3 and 6 months after the treatment onset (M3 and M6). D–30, D–15, D1 and D15 are the visits setting up the treatment.
Ankle-foot orthosis group
The ankle-foot orthosis (AFO) was a rigid ankle brace constructed of lightweight polypropylene-based thermoplastic material in the shape of an “L”, made in the Orthosis Preparation Unit, Henri Mondor University Hospitals (Créteil, France). To build the AFO, each participant underwent morphometric measurement of the leg and foot using plaster moulding at D–15, a visit for AFO delivery at D1, and a visit for potential orthosis shape adjustment at D15. The AFO was thus custom-made, with an upright portion behind the calf, attached to the leg with a strap, and a lower portion running under the foot, sufficiently thin to fit inside shoes. The smooth “L” shape of the AFO design provided relative rigidity to the ankle, with a fixed position at approximately 0° of dorsiflexion.
Semi-implanted selective functional electrical stimulation group
This study used the semi-implanted dropped foot stimulator (STIMuSTEP) developed by the University of Twente (Enschede, the Netherlands) and manufactured by Finetech Medical Ltd (Welwyn Garden City, UK) (24). This stimulator provides double neurostimulation onto 2 channels that transmit electrical pulses to the deep and the superficial branches of the common peroneal nerve. This device comprises an implanted passive receiver that catches stimulation pulses coming from an external controller strapped to the leg over the receiver, via close-coupled radio telemetry (11, 22–28). There are 2 epineural electrodes (a 9×2.75×0.8-mm assembly with a 1-mm diameter separated by 5 mm) inserted around the motor fascicles of the superficial peroneal branch (to peroneus brevis and longus) and the deep peroneal branch (to tibialis anterior, hallux and common toe extensors), sparing the sensory collaterals in order to minimize paresthesias. The pulse repetition rate is 30 Hz on each channel, the pulse width is set at 300 μs and the stimulation pulses used are voltage driven. The stimulation is triggered using a pressure-sensitive footswitch placed in the shoe. In the SIS-FES group, an external 1-channel-FES device (ODFS® III, Odstock Medical Ltd, Salisbury, UK) was provided to the participant from 2 months before the treatment onset (M–2) until D–30, for familiarization with the FES concept. The feasibility of using the FES technology in everyday life was assessed by the neurosurgeon investigator through a questionnaire at D–30. In addition, the participant underwent a preoperative anaesthesiology consultation at D–30, surgical implantation of the device at D–15, device activation and setting at the D1 visit, and stimulation intensity adjustment for both channels at the D15 visit.
Assessments
All participants were evaluated 2 months before treatment onset (visit M–2) using Perry’s modified walking functional categories (MWFC) (32), the functional independence measure (FIM) (33) and the neuropathic pain diagnostic questionnaire (DN4) (34). They underwent gait analysis at comfortable speed with shoes without and with assistive device AFO or SIS-FES (conditions OFF and ON) at M–2, M3 and M6, the latter 2 visits being 3 and 6 months after treatment onset. Gait was analysed in the laboratory using a motion capture system (10 cameras 4 MPx, Cortex software package, Motion Analysis Corporation, Santa Rosa, CA, USA) with 6 force plates (BERTEC Corporation, Columbus, OH, USA). The trajectories of 26 markers placed on anatomical landmarks using the Helen Hayes marker set (35) were collected (sampling frequency, 100 Hz) and filtered using a 4th order zero-lag Butterworth low pass filter, with a 6 Hz cut-off frequency (36, 37). At least 8 gait cycles for each lower limb were used for kinematic analysis. Eleven key parameters from the kinematic analysis were computed at comfortable walking speed: 1: walking speed, 2: cadence, 3: paretic step length, 4: non-paretic step length, and, for the paretic limb only during its swing phase, 5: maximal ankle dorsiflexion, 6: maximal ankle inversion, 7: maximal knee flexion, 8: maximal hip flexion, and the mean angle speed from the position at toe-off to the maximal 9: ankle dorsiflexion, 10: knee flexion and 11: hip flexion. The primary outcome, comfortable walking speed assessed in the laboratory, was calculated as the mean velocity of the sacrum along the room X-axis (progression of walk) averaged over a minimum of 8 gait cycles (38). All kinematic criteria were calculated using the International Society of Biomechanics (ISB) recommendations (35, 39).
Statistical analysis
The sample size was calculated assuming α (type I error) = 0.05, power (1–β) = 0.80, participation discontinuation = 0.20 and equal sample sizes in the 2 groups. The sample size equation was as follows (40):
n = 1.2* 7.85[(R + 1)–p2(R² + 1)]/p2(1–R)²;
where n = sample size in each of the groups; p1 = event rate in the treatment group; p2 = event rate in the control group; R = risk ratio (p1/p2). p1 and p2 were estimated from the clinical data in Taylor et al. (41). The study estimated a 25% event rate in the AFO group (p2 = 0.25) and determined that the clinically important difference to detect is a 40% increase with the SIS-FES intervention (p1 = 0.65).
The calculated sample size was 24.027 and was therefore set at 25 per group. Descriptive statistics for quantitative variables (age, time since onset, FIM, DN4 scores, walking speed, all other kinematic parameters) used the mean values and standard deviations (SDs) based on conditions of normality (Shapiro-Wilks). Analyses of variance (ANOVA) were used to compare the effects of 3 factors: group (AFO, SIS-FES), condition (OFF, ON) and visit (M–2, M3, M6) on dependent variables.
The study defined 4 types of effects of AFO and SIS-FES, which were explored in 4 different ways.
- The orthotic effect referred to the immediate changes in gait that occur after donning or switching on the device, explored by the interaction group*condition at M3 and M6. The main objective of the study was to compare the orthotic effects of AFO and SIS-FES on comfortable walking speed (primary outcome) at M3.
- The training effect, which may occur above and beyond that immediate orthotic effect, was assessed after a period of wearing the device, through the interaction group*visit in the ON condition at M3 and M6.
- The therapeutic effect refers to changes in walking seen without wearing the device, explored by the interaction group*visit in the OFF condition at M3 and M6.
- The combined effect refers to the changes in walking over time, encompassing both orthotic and training effects, and measuring by the interaction group*visit, using M–2 in OFF, and M3 and M6 in ON.
If overall comparisons revealed any significant difference across groups, conditions or visits, pairwise within- and between-factor comparisons were performed using Bonferroni corrections. If overall comparisons revealed no significant difference across groups, the data were pooled to analyse the effects of visit or condition in the whole sample of recruited participants. All statistical analyses were conducted with Statistica 7.0 software package (StatSoft, Inc., Tulsa, OK, USA). A p-value of 0.05 was used for statistical significance.
RESULTS
Participants
During the 3-year inclusion period, a total of 52 patients were screened among the 4 recruiting centres; however, only 27 participants could be included in the study, as described in Fig. 1. These 27 subjects with chronic spastic paresis (5 women; age 46 ± 14 years, mean ± SD; delay post-lesion, 9 ± 11 years, Table II) were randomized into the AFO group (n = 13) and the SIS-FES group (n = 14). Two patients (1 per group) interrupted their participation before D–15 and 1 patient in the SIS-FES group left the study before M3 (Fig. 1). One patient in the AFO group missed the M3 assessment. The results for 24 subjects are reported hereafter (12 patients per group). All patients in the SIS-FES group were able to use FES technology according to the neurosurgeon’s opinion at D–30 after using the external FES device for 1 month. No adverse events were reported in either group.
Orthotic effects: primary outcome
All kinematic data are shown in Table III.
Between-group effects
There was no between-group difference in change in the primary outcome, i.e. in the change in comfortable walking speed from OFF to ON, at M3 (Table III, Fig. 2A). In fact, between-group comparisons showed similar orthotic effects OFF vs ON on speed, cadence, step length, and ankle and knee amplitude (ANOVA, condition*group interaction, ns; Table IV; Fig. 2A). However, changes in opposite directions depending on the group were observed from OFF to ON in terms of ankle dorsiflexion speed, which decreased with AFO and remained stable with SIS-FES (ANOVA, condition*group interaction, p = 0.04; post-hoc Bonferroni, AFO: OFF, 11.4 ± 9.1°/s, ON, 4.6 ± 4.7°/s, p = 0.02; SIS-FES: OFF, 13.9 ± 8.8°/s, ON, 13.7 ± 8.8°/s, ns).
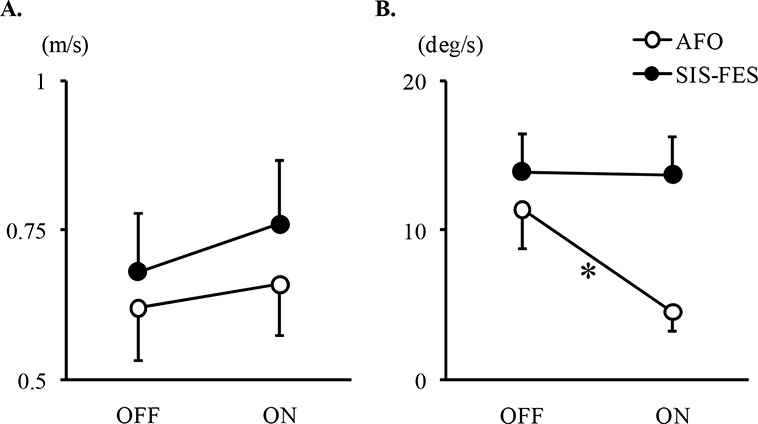
Fig. 2. Mean comfortable walking speed and mean dorsiflexion speed in early swing. Results expressed as mean±standard error of (A, primary outcome) the mean comfortable speed at month 3 and (B) the mean dorsiflexion speed calculated in early swing from the peak plantar flexion to the peak dorsiflexion, in the paretic limb using ankle-foot orthosis (AFO, n = 12) or semi-implanted selective functional electrical stimulation (SIS-FES, n = 11), without and with the device use (OFF and ON). Participants walked with shoes. The 2 gait assessments, M3 and M6, have been averaged in (B). *Post-hoc Bonferroni, p<0.05.
Pooled groups analysis
Both groups and visits (M3, M6) pooled, the device-associated kinematic changes (ANOVA, OFF vs ON, p < 0.05) included increases in gait speed ( OFF, 0.69 ± 0.28 m/s; ON, 0.76 ± 0.30 m/s, p < 0.001), cadence (OFF, 1.36 ± 0.28 step/s; ON, 1.42 ± 0.28 step/s, p < 0.001), paretic step length (OFF, 0.52 ± 0.13 m; ON, 0.55 ± 0.13 m, p < 0.001), non-paretic step length (OFF, 0.46 ± 0.15 m; ON, 0.49 ± 0.16 m, p = 0,007), maximal ankle dorsiflexion (OFF, –7 ± 7°; ON, –1 ± 7°, p < 0.001), maximal ankle inversion (OFF, 15 ± 9°; ON, 10 ± 8°, p < 0.001), but a decrease in maximal knee flexion (OFF, 40 ± 15°; ON, 38 ± 14°, p = 0.007).
Training effect
There was no between-group or intra-group difference in changes in the ON condition at M6 compared with M3 (ANOVA, visit*group interaction, ns; Table IV).
Therapeutic effect
There was no between-group difference for changes in the OFF condition from M–2 to M3 or M6 (ANOVA, visit*group interaction, ns; Table IV), although there was a numerical 3° reduction in dorsiflexion in the AFO group. Both groups pooled, peak knee flexion increased slightly in the OFF condition between M–2 and M3, with a sustained change at M6 (ANOVA, visit effect, p < 0.01; post-hoc Bonferroni, M–2, 38 ± 15°, M3, 40 ± 14°, p = 0.03; M6, 40 ± 15°, p = 0.03). Other parameters remained unchanged in the OFF condition at M–2, M3 and M6.
Combined effect
There was no between-group difference (ANOVA, condition*visit*group interaction, ns; Table IV). Both groups combined, comparison between OFF data at baseline (M–2) and data in the ON condition at M3 and M6 showed significant changes (ANOVA, condition*visit interaction, p < 0.05) based on increases in cadence (post-hoc Bonferroni, M–2 OFF vs M3 ON, + 0.08 ± 0.14 step/s, p = 0.02) and maximal dorsiflexion ( + 6 ± 6°, p < 0.001) at M3, which persisted at M6 (p < 0.05). Increases in gait speed (post-hoc Bonferroni, M–2 OFF vs M6 ON, + 0.10 ± 0.18 m/s, p = 0.03), in mean knee flexion speed ( + 11 ± 27°/s, p = 0.04) and a decrease in maximal ankle inversion (–3 ± 5°, p = 0.04) were observed at M6 only, both groups combined.
DISCUSSION
Main findings
In this randomized controlled study, SIS-FES (a functional electrical stimulation device with dual stimulation implanted around the 2 branches of the common peroneal nerve) and AFO used over 6 months produced similar effects on walking kinematics, particularly in peak ankle dorsiflexion and varus during swing phase, walking velocity, cadence and step length, in patients with chronic hemiparesis, except for ankle dorsiflexion speed in swing, which was reduced by half with AFO, while it was preserved when using SIS-FES. In the OFF condition after 6 months of use, the only benefit was a small 2° increase in knee flexion at swing with both devices. The lack of deterioration in dorsiflexion speed, and therefore potential advantage in stimulating muscle plasticity by providing repeated small phasic plantar flexor stretch at each swing phase, may favour the use of SIS-FES, particularly in the long-term. These findings should be confirmed in future long-term studies.
Tools for effective dorsiflexion support during walking
When wearing or switching on the device, the contribution of SIS-FES was not different from that of AFO with respect to improving paretic ankle position during the swing phase (dorsiflexion improved by 6° and varus corrected by 7° on average) and walking velocity (10% increase), with bilaterally longer and faster steps during comfortable walking. These findings corroborate data from previous randomized controlled trials with conventional FES (10, 13, 14). Greater improvements with implanted FES vs AFO might be expected on ankle plantar flexion power and propulsion in faster walkers (i.e. speed > 0.8 m/s), as suggested in a previous non-randomized trial (27, 28). Of note, the present participants walked at a comfortable speed of less than 0.7 m/s on average, which is mechanically associated with very low need for ankle power during push off (i.e. propulsion) in normal gait (42). Finally, there was no additional benefit across time (i.e. no training or therapeutic effects), except for the slight increase in knee flexion at swing in the OFF condition at M3 and M6. This study thus does not bring any argument in favour of any compelling therapeutic effects of these devices, at least over 6 months of use, beyond symptomatic assistive effects. In fact, the reduction in dorsiflexion speed during AFO use might increase concerns about the risk of deteriorating the capacity to actively dorsiflex the foot with long-term AFO use (see below).
Questioning permanent passive assistance in hemiparesis
The interest in continued use of assistive devices in hemiparesis may be questioned. Positive therapeutic effects of AFO and FES on walking speed after 4–30 weeks of use have been reported in the literature (12–14, 21), in contrast with the current findings. This discrepancy is possibly related to low statistical power in the current small cohort analysis. However, randomized controlled studies have not reported on ankle kinematics. The current study reports a 50% reduction in dorsiflexion velocity in early swing associated with 6-month use of AFO, together with a numeric 3° loss (from –6° to –9°) in active dorsiflexion during swing when not using the AFO after 6 months of use. This suggests that AFOs might limit active ankle movement restoration, which could be deleterious in the long run. Indeed, it has already been shown that the opposite intervention, i.e. applying torque perturbation during walking, leads to increased dorsiflexor activation (43). In addition, AFO-related ankle hypo-mobilization may contribute to worsening muscle and tendon architecture changes and, consequently, passive and active range of motion (44–46).
In contrast, SIS-FES, which allows free mobility of the ankle, did not decelerate dorsiflexion. A previous study reported an increase in tibialis anterior activity during voluntary isometric contraction, demonstrating some form of motor recovery after 3 months of FES use (18). In addition, recent open-label reports indicate decreased echogenicity and increased thickness in tibialis anterior as well as positive change in cortical activity after long-term implanted FES use (47, 48). Therefore this technique might promote both muscle and neural plasticity.
Study limitations
The small size of the sample limits generalization of the current findings. During the 3-year inclusion period, the expected number of subjects (n = 50) was not reached. Another potential limitation was the lack of control in the rate of everyday device use at home. The set-up is similar with AFO and with SIS-FES in terms of complexity and duration, with elements to mount on the leg and inside the shoe (SIS-FES setting being pre-configured). No indication of different daily use between groups was suspected from the available data. Lastly, the study did not include control for associated rehabilitation care, even though it was requested at inclusion that such care be left unchanged during the study.
CONCLUSION
This study promotes AFO and SIS-FES primarily as deficit compensators, not as treatments of ankle dorsiflexion impairment during gait, since no kinematic benefit was observed in the OFF condition after 6 months of use, except for a slight improvement in knee flexion at swing. However, attention must be drawn to the active dorsiflexion capacity, which may be impaired by AFO that corrects ankle movement to the expense of reduced participation of the command and reduced plantar flexor stretch at each step. The SIS-FES may thus be more capable of preserving the ability to raise the foot in the long term. Further research could explore the long-term effects (several years) of rehabilitation with implanted FES compared with rehabilitation without using the device.
ACKNOWLEDGEMENTS
The authors would like to thank the following investigator centres at Henri Mondor University Hospitals (Créteil, France): the Department of Neurorehabilitation and its Orthosis Manufacturing Unit, the Department of Functional Explorations and the Clinical Research Unit, and the following recruiting centres: the Fernand Widal-Lariboisière Hospitals (Paris, France), the Saint-Maurice Hospitals (Saint Maurice, France) and the Coubert Rehabilitation Center (Coubert, France).
REFERENCES
- Gracies JM. Pathophysiology of spastic paresis. I: Paresis and soft tissue changes. Muscle Nerve 2005; 31: 535–551. DOI: 10.1002/mus.20284
- Gracies JM. Pathophysiology of spastic paresis. II: Emergence of muscle overactivity. Muscle Nerve 2005; 31: 552–571. DOI: 10.1002/mus.20285
- Ruderman RJ, Bajema SL, McDowell JF. A universal temporary ankle-foot orthosis. Phys Ther 1973; 53: 151–152. DOI: 10.1093/ptj/53.2.151
- Hesse S, Luecke D, Jahnke MT, Mauritz KH. Gait function in spastic hemiparetic patients walking barefoot, with firm shoes, and with ankle-foot orthosis. Int J Rehabil Res 1996; 19: 133–141. DOI: 10.1097/00004356-199606000-00004
- Ferreira LA, Neto HP, Grecco LA, Christovão TC, Duarte NA, Lazzari RD, et al. Effect of ankle-foot orthosis on gait velocity and cadence of stroke patients: a systematic review. J Phys Ther Sci 2013; 25: 1503–1508. DOI: 10.1589/jpts.25.1503
- Tyson SF, Sadeghi-Demneh E, Nester CJ. A systematic review and meta-analysis of the effect of an ankle-foot orthosis on gait biomechanics after stroke. Clin Rehabil 2013; 27: 879–891. DOI: 10.1177/0269215513486497
- Liberson WT, Holmquest HJ, Scot D, Dow M. Functional electrotherapy: stimulation of the peroneal nerve synchronized with the swing phase of the gait of hemiplegic patients. Arch Phys Med Rehabil 1961; 42: 101–105.
- Lyons GM, Sinkjaer T, Burridge JH, Wilcox DJ. A review of portable FES-based neural orthoses for the correction of drop foot. IEEE Trans Neural Syst Rehabil Eng 2002; 10: 260–279. DOI: 10.1109/TNSRE.2002.806832
- Gil-Castillo J, Alnajjar F, Koutsou A, Torricelli D, Moreno JC. Advances in neuroprosthetic management of foot drop: a review. J Neuroeng Rehabil 2020; 17: 46. DOI: 10.1186/s12984-020-00668-4
- Sheffler LR, Hennessey MT, Naples GG, Chae J. Peroneal nerve stimulation versus an ankle foot orthosis for correction of footdrop in stroke: impact on functional ambulation. Neurorehabil Neural Repair 2006; 20: 355–360. DOI: 10.1177/1545968306287925
- Kottink AI, Hermens HJ, Nene AV, Tenniglo MJ, van der Aa HE, Buschman HP, et al. A randomized controlled trial of an implantable 2-channel peroneal nerve stimulator on walking speed and activity in poststroke hemiplegia. Arch Phys Med Rehabil 2007; 88: 971–978. DOI: 10.1016/j.apmr.2007.05.002
- Morone G, Fusco A, Di Capua P, Coiro P, Pratesi L. Walking training with foot drop stimulator controlled by a tilt sensor to improve walking outcomes: a randomized controlled pilot study in patients with stroke in subacute phase. Stroke Res Treat 2012; 2012: 523564. DOI: 10.1155/2012/523564
- Everaert DG, Stein RB, Abrams GM, Dromerick AW, Francisco GE, Hafner BJ, et al. Effect of a foot-drop stimulator and ankle-foot orthosis on walking performance after stroke: a multicenter randomized controlled trial. Neurorehabil Neural Repair 2013; 27: 579–591. DOI: 10.1177/1545968313481278
- Kluding PM, Dunning K, O’Dell MW, Wu SS, Ginosian J, Feld J, et al. Foot drop stimulation versus ankle foot orthosis after stroke: 30-week outcomes. Stroke 2013; 44: 1660–1669. DOI: 10.1161/STROKEAHA.111.000334
- Bethoux F, Rogers HL, Nolan KJ, Abrams GM, Annaswamy TM, Brandstater M, et al. The effects of peroneal nerve functional electrical stimulation versus ankle-foot orthosis in patients with chronic stroke: a randomized controlled trial. Neurorehabil Neural Repair 2014; 28: 688–697. DOI: 10.1177/1545968314521007
- Bethoux F, Rogers HL, Nolan KJ, Abrams GM, Annaswamy T, Brandstater M, et al. Long-term follow-up to a randomized controlled trial comparing peroneal nerve functional electrical stimulation to an ankle foot orthosis for patients with chronic stroke. Neurorehabil Neural Repair 2015; 29: 911–922. DOI: 10.1177/1545968315570325
- Burridge JH, Taylor PN, Hagan SA, Wood DE, Swain ID. The effects of common peroneal stimulation on the effort and speed of walking: a randomized controlled trial with chronic hemiplegic patients. Clin Rehabil 1997; 11: 201–210. DOI: 10.1177/026921559701100303
- Sabut SK, Sikdar C, Mondal R, Kumar R, Mahadevappa M. Restoration of gait and motor recovery by functional electrical stimulation therapy in persons with stroke. Disabil Rehabil 2010; 32: 1594–1603. DOI: 10.3109/09638281003599596.
- Ghédira M, Albertsen IM, Mardale V, Gracies JM, Bayle N, Hutin É. Wireless, accelerometry-triggered functional electrical stimulation of the peroneal nerve in spastic paresis: a randomized, controlled pilot study. Assist Technol 2017; 29: 99–105. DOI: 10.1080/10400435.2016.1214933.
- Prenton S, Hollands KL, Kenney LP. Functional electrical stimulation versus ankle foot orthoses for foot-drop: a meta-analysis of orthotic effects. J Rehabil Med 2016; 48: 646–656. DOI: 10.2340/16501977-2136
- Prenton S, Hollands KL, Kenney LPJ, Onmanee P. Functional electrical stimulation and ankle foot orthoses provide equivalent therapeutic effects on foot drop: a meta-analysis providing direction for future research. J Rehabil Med 2018; 50: 129–139. DOI: 10.2340/16501977-2289
- Kottink AI, Hermens HJ, Nene AV, Tenniglo MJ, Groothuis-Oudshoorn CG, IJzerman MJ. Therapeutic effect of an implantable peroneal nerve stimulator in subjects with chronic stroke and footdrop: a randomized controlled trial. Phys Ther 2008; 88: 437–448. DOI: 10.2522/ptj.20070035
- Taylor PN, Wilkinson Hart IA, Khan MS, Slade-Sharman DE. Correction of footdrop due to multiple sclerosis using the STIMuSTEP implanted dropped foot stimulator. Int J MS Care 2016; 18: 239–247. DOI: 10.7224/1537-2073.2015-038
- Holsheimer J, Bultstra G, Verloop AJ, van der Aa HE, Hermens HJ. Implantable dual channel peroneal nerve stimulator. Proceedings Ljubljana FES conference, Ljubljana, Slovenia, 1993; pp. 42–44.
- van der Aa HE, Bultstra G, Verloop AJ, Kenney L, Holsheimer J, Nene A, et al. Application of a dual channel peroneal nerve stimulator in a patient with a “central” drop foot. Acta Neurochir Suppl 2002; 79: 105–107. DOI: 10.1007/978-3-7091-6105-0_23
- Kottink AI, Buschman HP, Kenney LP, Veltink PH, Slycke P, Bultstra G, et al. The sensitivity and selectivity of an implantable two-channel peroneal nerve stimulator system for restoration of dropped foot. Neuromodulation 2004; 7: 277–283. DOI: 10.1111/j.1094-7159.2004.04213.x
- Schiemanck S, Berenpas F, van Swigchem R, van den Munckhof P, de Vries J, Beelen A, et al. Effects of implantable peroneal nerve stimulation on gait quality, energy expenditure, participation and user satisfaction in patients with post-stroke drop foot using an ankle-foot orthosis. Restor Neurol Neurosci 2015; 33: 795–807. DOI: 10.3233/RNN-150501
- Berenpas F, Schiemanck S, Beelen A, Nollet F, Weerdesteyn V, Geurts A. Kinematic and kinetic benefits of implantable peroneal nerve stimulation in people with post-stroke drop foot using an ankle-foot orthosis. Restor Neurol Neurosci 2018; 36: 547–558. DOI: 10.3233/RNN-180822
- Ghédira M, Pradines M, Mardale V, Gracies JM, Bayle N, Hutin E. Quantified clinical measures linked to ambulation speed in hemiparesis. Top Stroke Rehabil 2021; 6: 1–12. DOI: 10.1080/10749357.2021.1943799
- Ramsay JW, Wessel MA, Buchanan TS, Higginson JS. Poststroke muscle architectural parameters of the tibialis anterior and the potential implications for rehabilitation of foot drop. Stroke Res Treat 2014; 2014: 948475. doi:10.1155/2014/948475.
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 20; 370: 1453–1457. DOI: 10.1016/S0140-6736(07)61602-X
- Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke 1995; 26: 982–989. DOI: 10.1161/01.str.26.6.982
- Keith RA, Granger CV, Hamilton BB, Sherwin FS. The functional independence measure: a new tool for rehabilitation. Adv Clin Rehabil 1987; 1: 6–18.
- Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain 2005; 114: 29–36. DOI: 10.1016/j.pain.2004.12.010
- Wu G, Siegler S, Allard P, Kirtley C, Leardini A, Rosenbaum D, et al; Standardization and Terminology Committee of the International Society of Biomechanics. ISB recommendation on definitions of joint coordinate system of various joints for the reporting of human joint motion – part I: ankle, hip, and spine. International Society of Biomechanics. J Biomech 2002; 35: 543–548. DOI: 10.1016/s0021-9290(01)00222-6
- Gold. B, Radar C. Digital processing of signals. New York: McGraw-Hill Co.; 1969.
- Winter DA, Sidwall HG, Hobson DA. Measurement and reduction of noise in kinematics of locomotion. J Biomech 1974; 7: 157–159. DOI: 10.1016/0021-9290(74)90056-6
- Winter DA. The biomechanics and motor control of human gait. Ontario, Canada: University of Waterloo; 1987.
- Kadaba MP, Ramakrishnan HK, Wootten ME. Measurement of lower extremity kinematics during level walking. J Orthop Res 1990; 8: 383–392. DOI: 10.1002/jor.1100080310
- Schulz KF, Grimes DA. Sample size calculations in randomised trials: mandatory and mystical. Lancet 2005; 9–15; 365: 1348–1353. DOI: 10.1016/S0140-6736(05)61034-3
- Taylor PN, Burridge JH, Dunkerley AL, Wood DE, Norton JA, Singleton C, et al. Clinical use of the Odstock dropped foot stimulator: its effect on the speed and effort of walking. Arch Phys Med Rehabil 1999; 80: 1577–1583. DOI: 10.1016/s0003-9993(99)90333-7
- Winter DA. Biomechanical motor patterns in normal walking. J Mot Behav 1983; 15: 302–330. DOI: 10.1080/00222895.1983.10735302
- Blanchette AK, Noël M, Richards CL, Nadeau S, Bouyer LJ. Modifications in ankle dorsiflexor activation by applying a torque perturbation during walking in persons post-stroke: a case series. J Neuroeng Rehabil 2014; 11: 98. DOI: 10.1186/1743-0003-11-98
- Tabary JC, Tabary C, Tardieu C, Tardieu G, Goldspink G. Physiological and structural changes in the cat’s soleus muscle due to immobilization at different lengths by plaster casts. J Physiol 1972; 224: 231–244. DOI: 10.1113/jphysiol.1972.sp009891
- Jalal N, Gracies JM, Zidi M. Mechanical and microstructural changes of skeletal muscle following immobilization and/or stroke. Biomech Model Mechanobiol 2020; 19: 61–80. DOI: 10.1007/s10237-019-01196-4
- Maganaris CN, Reeves ND, Rittweger J, Sargeant AJ, Jones DA, Gerrits K, et al. Adaptive response of human tendon to paralysis. Muscle Nerve 2006; 33: 85–92. DOI: 10.1002/mus.20441
- Berenpas F, Weerdesteyn V, Geurts AC, van Alfen N. Long-term use of implanted peroneal functional electrical stimulation for stroke-affected gait: the effects on muscle and motor nerve. J Neuroeng Rehabil 2019; 16: 86. DOI: 10.1186/s12984-019-0556-2
- Thibaut A, Di Perri C, Heine L, Moissenet F, Chantraine F, Schreiber C, et al. Neuroplastic changes mediate motor recovery with implanted peroneal nerve stimulator in individuals with chronic stroke: an open-label multimodal pilot study. Ann Phys Rehabil Med 2021; 64: 101358. DOI: 10.1016/j.rehab.2020.01.004