ABSTRACT
Objective: To assess gripforce in children with a C5 and C6 neonatal brachial plexus palsy, as it may affect hand use. Applying classic innervation patterns, gripforce should not be affected, as hand function is not innervated by C5 or C6. This study compares gripforce in children with a neonatal brachial plexus palsy with that in a healthy control group, and assesses correlations with hand sensibility, bimanual use and external rotation.
Methods: A total of 50 children with neonatal brachial plexus palsy (mean age 9.8 years) and 25 controls (mean age 9.6 years) were investigated. Nerve surgery had been performed in 30 children, and 20 children had been treated conservatively. Gripforce of both hands was assessed using a Jamar dynamometer. Sensibility of the hands was assessed with 2-point discrimination and Semmes-Weinstein monofilaments. External rotation was assessed using the Mallet score. Bimanual use was measured by using 1 of 3 dexterity items of the Movement Assessment Battery for Children-2. The affected side of the neonatal brachial plexus palsy group was compared with the non-dominant hand of the control group using 1-way analysis of variance (ANOVA), χ2 and Mann–Whitney tests.
Results: The mean gripforce of the affected non- dominant hand of children with neonatal brachial plexus palsy was reduced compared with healthy controls (95 N and 123 N, respectively, with p = 0.001). The mean gripforce of the non-dominant hand in the control group was 92% of that of the dominant hand, while it was only 76% in the neonatal brachial plexus palsy group (p = 0.04). There was no relationship between gripforce reduction and sensibility, bimanual use or shoulder external rotation.
Discussion: The gripforce in neonatal brachial plexus palsy infants with a C5 and C6 lesion is lower than that of healthy controls, although classic interpretation of upper limb innervation excludes this finding. The reduction in gripforce in upper neonatal brachial plexus palsy lesions is not widely appreciated as a factor inherently compromising hand use. The reduction in gripforce should be taken into consideration in planning the type of rehabilitation and future activities.
Key words: neonatal brachial plexus palsy; sensibility; dominant hand; gripforce; external rotation; dexterity.
Accepted Jun 8, 2021; Epub ahead of print Jun 16, 2021
J Rehabil Med 2021; 53: jrm00219
Correspondence address: Sonja M. Buitenhuis, Department of Neurosurgery, Leiden University Medical Center, Leiden, The Netherlands. E-mail: s.m.buitenhuis@lumc.nl
LAY ABSTRACT
Children with a shoulder nerve lesion caused at birth, use the hand at that side in a limited way. This is odd, as hand function is not controlled by nerves of the shoulder plexus. To get more information about the hand use, we investigated the gripforce and compared this with healthy children. This objective comparison has not been carried out before. Fifty children with comparable shoulder nerve lesion and twenty five controls were investigated. Gripforce of the affected hand was 23 % lower than the gripforce of the hand of the controls. This finding contradict previous research. Also the often limited external rotation of the shoulder in the group with nerve lesion could not be correlated with the diminished gripforce. Diminished gripforce is not widely appreciated as a factor compromising hand use. This reduction should be taken into consideration in planning the type of rehabilitation and future activities.
INTRODUCTION
Neonatal brachial plexus palsy (NBPP) is a nerve traction injury that occurs during birth. The most common type involves a lesion of the 2 upper spinal nerves, C5 and C6. In more severe cases, spinal nerves C7, C8 and T1 are also involved (1). Classic anatomical innervation schemes indicate that C5 mainly innervates the deltoid, supraspinatus and infraspinatus muscles, whereas the biceps, brachialis and brachioradialis muscles are innervated mainly by C6 (2). An upper lesion should, therefore, only affect shoulder functions and elbow flexion. Our clinical observation is, however, that children with an upper NBPP use their hand less often, and the dexterity of the hand seems reduced. Anecdotally, parents affirm this observation. A satisfactory explanation for these phenomena is difficult to provide. After all, hand function, in itself, should not be affected in upper plexus injuries, because the muscles of the hand are innervated by the lower nerves of the brachial plexus, C8 and T1. Three factors could theoretically affect hand use: (i) a diminished positioning of the hand in space, (ii) a reduced sensibility, and (iii) gripforce reduction. Research has shown that recovery of glenohumeral external rotation is limited after conservative management or nerve surgery, reaching beyond the sagittal plane in only 20% of patients (3). Limitations in external positioning may affect the development of the preferred hand for writing and playing (4). Sensibility of the thumb and index finger in children with an upper plexus lesion (whether treated surgically or conservatively) is also reduced (5, 6), which is correlated with reduced dexterity (5). Some studies have been performed in children with an upper palsy, to explore whether the use of the hand is reduced because of a reduction in gripforce. The applied methodologies in these studies, however, leave doubts as to the value of the results. Namely, the affected side was compared with the non-affected side of the individual child (4, 7, 8). Regardless of the outcome, this type of comparison does not discriminate whether findings are caused by a relative increase in gripforce in the non-affected side, by hand dominance or by a reduction in intrinsic gripforce of the affected hand. In addition, the criteria used to define a reduction in gripforce were chosen quite arbitrarily and were based on measurements in adults. To overcome these issues, potential gripforce differences can be addressed only in the setting of a comparison with a healthy control group of the same age.
The current study compared the gripforce of the hand in children with an upper NBPP with that of healthy controls. In addition, gripforce was correlated with hand sensibility, dexterity/bimanual use and glenohumeral external rotation, in order to gain a better understanding of the bimanual use of the hand.
PATIENTS AND METHODS
A cross-sectional investigation design of patients with NBPP was used. Fifty children with an upper NBPP and 25 healthy children were recruited for the study. Ages of both groups ranged between 7 and 12 years. The children with NBPP had been examined on a regular basis from an early age at our tertiary referral clinic (Nerve Center of the Leiden University Medical Center, The Netherlands). The diagnosis of NBPP was based on the obstetric history and the neurological examination, and additional electromyography examination performed between the ages of 4 and 6 weeks (9). Nerve surgery was performed in 30 children (60%) in early infancy, while 20 had been treated conservatively. Based on the neurological examination, all children only had a lesion of the C5 and C6 spinal nerves. At the first visit to our clinic, all participants had a normal hand function, normal elbow extension based on active triceps muscle and active wrist extension at least against resistance. Hence, these children were diagnosed with a C5–C6 lesion, with intact C7–C8–T1 functions. The children who were conservatively treated showed recovery of elbow flexion with active biceps muscles at 3–6 months of age.
The indication for nerve surgery has been extensively described (1). Children who were operated upon underwent magnetic resonance imaging (MRI) myelography to assess root avulsion injuries. During the operation, surgical inspection and direct nerve stimulation were performed to confirm the clinical diagnosis. To restore C6 function, grafting from C6 to the anterior division of the superior trunk (ADST) was performed in 23 of the 30 infants. Of the remaining 7 patients, 5 had a medial pectoral nerve to musculocutaneous nerve transfer. In one surgically treated patient, accessory to suprascapular nerve transfer was the sole procedure, and in one other patient surgery was limited to neurolysis. In these 7 children, the nerve pathway from C6 to the ADST (containing the sensory fibres of the C6 dermatome) was in continuity. This sub-group of 7 patients was additionally compared with the 23 infants in whom C6 was grafted to the ADST.
The control group was recruited at the Montessori school at Voorburg, the Netherlands, by announcing the study on the school’s message board. All children who participated had a normal cognitive function and attended regular school (10).
Physical examination
Physical examination of all participants was performed by one physical therapist (SB) with a high level of experience of physical assessment and treatment of children in all age groups.
The gripforce of both hands was assessed with the Jamar dynamometer, according to a standard protocol (11). The child was sitting with the elbow and forearm resting on a table, with the wrist in a neutral position between pronation and supination. The shoulder was positioned in 0° anteflexion, 0° abduction and 0° external rotation. If this position was not possible due to lack of external rotation, the upper arm was held in a resting position in internal rotation. The dominant hand was tested first. The child was asked to squeeze the handles of the Jamar dynamometer as forcefully as possible. Three attempts at maximum force were recorded, and the mean of the 3 values was calculated. Before the 3 measurements were done, the children were instructed to do their utmost best to perform as well as they could. Also, during the testing we encouraged them to use the maximum of their abilities. The affected side was compared with the non-affected side within the NBPP group. In addition, the non-dominant affected side of the NBPP group was compared with the non-dominant hand of the control group.
The dominant hand was defined as the hand in which a child holds a pencil to write. A hand preference shift in the NBPP group was presumed to have occurred if a child with a right-sided lesion had left-hand dominance (12). When the dominant side was the affected side, children were excluded from analysis.
The sensibility of the hands was assessed with 2-point discrimination (2PD) (13) of the index finger and the Semmes-Weinstein Monofilament test (SW) (14) of the thumb of the non-dominant side in the NBPP group. External rotation was assessed using the relevant Mallet sub-score (15). A score of Mallet I signifies no active external rotation. Mallet II indicates < 0° active external rotation. Mallet III represents active external rotation between 0° and 20°. Mallet IV means > 20° active external rotation.
The combined use of both hands was measured by a single item from the 3 dexterity items of the Movement Assessment Battery for Children-2 (MABC-2), an internationally accepted and validated test for fine motor skills (16). For children aged 7, 8, 9 or 10 years, the specific bimanual task consisted of threading a wire through holes in a board. Children aged 11 or 12 years were instructed to construct a triangle with nuts and bolts in correspondence with MABC-2. This bimanual task was selected because it requires use of the affected hand. Children were not allowed to put either the wire or the triangle on the table, but were instructed to keep them in both hands. The time needed to finish the task was noted and converted to a standard score, and corrected for age using the MABC-2 manual. We have reported the sensibility and the dexterity results of the NBPP and control groups in a previous paper (5).
The study protocol was approved by the Medical Ethics Committee of Leiden University Medical Centre (ABR number 48977), and informed consent was given by the parents.
Statistical analysis
Analysis of variance (1-way ANOVA) was used for continuous outcome variables.
Categorical outcome variables were compared between groups using χ2 tests (exact tests if the expected counts were small). Where appropriate, a Mann–Whitney test was used instead of a t-test. The error level was set at p < 0.05. Data were analysed with SPSS Statistics for Windows, version 23 (IBM Corp. Armonk, NY, USA).
RESULTS
Patient details are shown in Table I. The affected (non-dominant) side in the NBPP group was compared with the non-dominant side of the control group, and the difference in gripforce was compared between the dominant and non-dominant hand within the NBPP group, respectively, within the control group. This analysis of the “affected non-dominant hand” concerned 28/30 surgically treated children and 14/20 conservatively treated children. (Table II). In the surgically treated group, a hand preference shift was found in 13/15 children (87%), while in the conservatively treated patients, a hand preference shift was found in 3/9 children (33%). This difference was statistically significant (p = 0.007). We cross-checked for sex or age as a confounder, as gripforce was shown to increase with age, and boys are usually stronger than girls (17). Our NBPP group was 0.3 years older than the control group, and sex did not influence the results, thereby ruling out these confounders.


The mean gripforce of the non-dominant affected hand was statistically significantly reduced in the NBPP group compared with the non-dominant hand of the controls (95 N and 123 N, respectively, with p = 0.001). The mean gripforce of the non-dominant hand in the control group was 92% of that of the dominant hand. The gripforce of the non-dominant hand of the NBPP group was 76% of that of the dominant hand (p = 0.04) (Fig. 1 and Table III). Gripforce did not differ statistically between the conservatively and surgically treated subgroups. The mean gripforce was 75% of the unaffected side after nerve grafting (n = 22); 81% after nerve transfer (n = 5); and 78% after conservative treatment (n = 14).

The gripforce of the non-injured dominant hand was reduced by10% in children who had undergone nerve surgery compared with controls (121 N vs 134 N; p = 0.20), and by 15% in children who shifted dominance (114 N vs 134 N; p = 0.13) (Table III).

In the MABC-2 bimanual use test, the children in the control group (n = 22) had a mean test score of 11.0, compared with a mean test score of 8.0 in the NBPP group (n = 50). The higher test score signifies that the controls perform the bimanual use test faster than the children with NBPP, which was statistically significant (p = 0.036). Due to a slight age difference between patients and controls, 31/50 (62%) of the children with NBPP performed the wire thread test compared with 20/22 (91%) of the controls. In addition, the wire thread test was analysed only to rule out different outcomes due to the difference between the wire thread test and triangle construction test. The time to thread the wire through holes in a board, was 29.9 s in the NBPP group (mean age 10.1 years) compared with 23.9 s in the control group (mean age 9.1 years). The time difference was also significant (p = 0.01).
Shoulder external rotation scores were Mallet grade I in 16 children with NBPP; Mallet II in 18; Mallet III in 12; and Mallet IV in 4.
No correlation was found between sensibility and gripforce. No correlation was found between gripforce and the bimanual use test (Pearson correlation coefficient: 0.092, p = 0.47). In addition, no correlation was found between gripforce (corrected for age) and the Mallet subscore for external rotation (p = 0.57).
DISCUSSION
The aim of this study was to analyse whether gripforce is reduced in children with an upper NBPP lesion, following the clinical observation that the affected hand is used less. This research question was supported by the observation of others that a shift of hand preference occurs in many children with NBPP (12).
In the current study, reduced gripforce was found in the affected side (at 76% of the gripforce of the unaffected side). In the healthy control group, the mean gripforce of the non-dominant side was 92% of that of the dominant hand. The findings in the control group match those of a previous report, in which a 94% ratio was found (11).
The current findings remove doubt regarding gripforce levels in upper NBPP lesions. Previously, it was stated that 50% of children with C5–C6 lesions have a reduced gripforce (4, 7). A Martin Vigori meter, consisting of a rubber bulb connected to a manometer, was used. The bulb had to be squeezed 3 times and the highest value for each hand was recorded. Gripforce was regarded as reduced when it was 20% less than the strength in the unaffected hand. A cut-off point of 20% was chosen based on gripforce measurements with a dynamometer in adults (18–20), which, in the context of children, is quite arbitrary. In another study another cut-off point was applied, namely more than 89% of the unaffected hand. It was found that only 18% of children with NBPP with a C5–C6 injury had a normal gripforce ratio (8). The discrepancies between studies (8, 18, 20), illustrate the effect of choosing different criteria on outcome, and create doubts as to its value.
The factors that cause a decrease in hand gripforce in NBPP with C5–C6 lesions remain to be determined.
A neuroanatomical explanation for the innervation of gripforce seems unlikely, as the long flexors of the fingers are innervated by C8 and T1, which should be normal in children with an upper trunk lesion. Indirectly, the innervation of wrist extension might play a role, as stable wrist extension is essential for a strong hand grip. Electrophysiological studies have shown that the nerve fibres innervating the extensor carpi radialis muscle arise from C5 and C6 (21). Reduced innervation of wrist extension could therefore contribute to a decrease of gripforce. However, in our experience, in lesions limited to the C5–C6 spinal nerves we never observe prominent reduction in wrist extension when we resect a neuroma of the superior trunk followed by nerve grafting. This implies that there is sufficient innervation from C7 and C8 to maintain a proper wrist extension with the extensor carpi radialis and ulnaris muscles (21). Overall, it therefore seems unlikely that neuroanatomical factors are involved in the reduction in gripforce in C5–C6 NBPP lesions.
An indirect explanation of the reduced gripforce of the hand on the affected side in children with NBPP might be the reduced use of the affected side, which may lead to a decreased force. The factors causing less use of the hand caused by impaired spatial positioning due to a limited shoulder function, and reduced dexterity due to reduced sensibility. These factors have been investigated, and it has been shown that the ability to incorporate the affected arm and hand in a co-ordinated movement pattern correlated with sensation and prehension of the hand, but not with shoulder and elbow function (22).
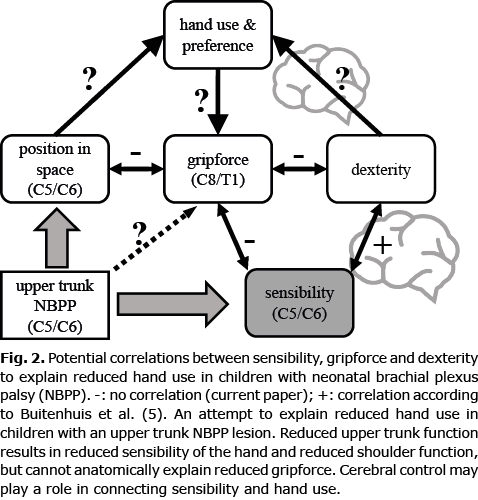
No statistical correlation was found between gripforce and sensibility, between gripforce and external rotation, or between gripforce and bimanual use. Due to the absence of such correlations, a direct relationship seems unlikely. One might cautiously conclude that other factors, which have, so far, not been defined or measured, play a causative role. One of these might be cerebral control, which is potentially disturbed in the development of central motor programmes. In clinical observations and fMRI data, evidence of changes in central control was found (23, 24). It was previously hypothesized that a reduced tactile input to the brain could explain diminished embedding of movement of the affected arm, which was coined “developmental apraxia” (25). Strombeck et al. (26) concluded considerable electromyography (EMG) changes observed in NBPP, even within fully recovered children. We previously assessed sensibility with 2PD and SW filaments, and found that the index finger is the most sensitive finger tested with the 2PD test, and the thumb with the SW (10), Sensibility of the thumb and index finger are essential to perform fine motor tasks, and it proved that these fingers showed reduced sensibility in the children with an upper trunk lesion. The absence of a correlation between gripforce and sensation in this study, does not exclude a role of sensation. Disruption of propriocepsis might be of more relevance, rather than the tactile sensation we tested (Fig. 2). For the execution of a fine coordinative movement, such as threading a wire through holes in a board, or constructing a triangle with nuts and bolts, both proprioceptive and tactile sensibility are more important than force.
In this series, the non-injured dominant hand had a 10–15% decreased gripforce compared with the dominant hand in controls, both in children who had undergone nerve surgery and in children who had a presumed dominance shift. These differences were not statistically significant, and may be a result of chance. However, we feel that this observation deserves further study, as the absence of statistical significance could also be caused by the relatively limited size of the current cohort. Not surprisingly, a dominance shift occurred frequently in surgically treated children who had a more severe nerve lesion than conservatively treated children. We hypothesize that a shift in hand dominance affects the dominant non-injured side at the cerebral levels of movement control, causing an additional disadvantage for learning bimanual activities. A cortical dominance shift has been described earlier in relation to speech development (27).
Finally, the current findings may be relevant for strategic choices in brachial plexus repair. Fascicles from the ulnar and median nerves are used as donors in transfers to the biceps and brachialis motor branch to reanimate elbow flexion in case of root avulsion in NBPP. Although the use of these fascicles might jeopardize hand function development, hand dysfunction was not found following the use of either the ulnar or median fascicle in a small series of 8 patients (28). In this series, however, only the affected hand was examined, and findings were not related to the non-affected side or healthy controls. Since we show that gripforce is reduced even in upper lesions, it might, in fact, be the case that more hand function is lost due to the application of this technique than is currently appreciated.
A limitation of the current study is that the participating children were followed at our tertiary referral clinic, and as a result, surgically treated children were over-represented in our sample. Surgical procedures were diverse, but gripforce did not statistically differ in different surgical groups. In addition, children with a good clinical recovery after surgery or conservative treatment usually do not have a long follow-up and are, therefore, under-represented in the current study. Another limitation of our study is that we did not systematically document whether the use of the hand was reduced, but rather documented it anecdotally when parents reported it during visits to our clinic. Future studies should include patient-reported outcome measures; for example, the Hand-Use-at-Home questionnaire to document the frequency of hand employment (29).
In summary, various explanations have been offered for the reduced hand use in NBPP children with an upper palsy (Fig. 2). In the current study, children appeared to have a reduced gripforce. This finding was not directly correlated with reduced sensibility or other factors.
More research is needed to fully understand the reduced hand usage and gripforce in upper trunk NBPP. It is advised to assess the dominant non-injured hand in future cohorts, and its role in dexterity and bimanual activities. This may ultimately provide clues for designing tailored physical or occupational therapy to improve hand usage.
CONCLUSION
Reduced gripforce of the hand was found in children with an upper neonatal brachial plexus lesion, which we hypothesize to be caused by reduced use of the hand, and reduced cerebral control. In addition, the non-injured hand had reduced grip force, especially in children with a presumed dominance shift, which may further impair their bimanual ability.
No relationship was found between gripforce and sensibility, bimanual use or shoulder external rotation function.
The authors have no conflicts of interest to declare.
REFERENCES
- Malessy MJ, Pondaag W. Obstetric brachial plexus injuries. Neurosurg Clin N Am 2009; 20: 1–14.
- Merle d’Aubigné R, Deburge A. Etiologie, évolution et pronostic des paralysies traumatiques du plexus brachial. Rev Chir Orthop 1967; 53: 23–42.
- Pondaag W, de Boer R, van Wijlen-Hempel MS, Hofstede-Buitenhuis SM, Malessy MJ. External rotation as a result of suprascapular nerve neurotization in obstetric brachial plexus lesions. Neurosurgery 2005; 57: 530–537.
- Krumlinde-Sundholm L, Eliasson AC, Forssberg H. Obstetric brachial plexus injuries: assessment protocol and functional outcome at age 5 years. DevMed Child Neurol 1998; 40: 4–11.
- Buitenhuis SM, Pondaag W, Wolterbeek R, Malessy MJA. Sensibility of the hand in children with conservatively or surgically treated upper neonatal brachial plexus lesion. Pediatr Neurol 2018; 86: 57–62.
- Anguelova GV, Malessy MJ, van Dijk JG. Sensory deficit in conservatively treated neonatal brachial plexus palsy patients. Pediatr Neurol 2016; 62: e1.
- Strombeck C, Krumlinde-Sundholm L, Forssberg H. Functional outcome at 5 years in children with obstetrical brachial plexus palsy with and without microsurgical reconstruction. Dev Med Child Neurol 2000; 42: 148–157.
- Kirjavainen M, Remes V, Peltonen J, Rautakorpi S, Helenius I, Nietosvaara Y. The function of the hand after operations for obstetric injuries to the brachial plexus. J Bone Joint SurgBr 2008; 90: 349–355.
- Malessy MJ, Pondaag W, Yang LJ, Hofstede-Buitenhuis SM, le CS, Van Dijk JG. Severe obstetric brachial plexus palsies can be identified at one month of age. PLoS One 2011; 6:e26193.
- Buitenhuis SM, Pondaag W, Wolterbeek R, Malessy MJA. Hand sensibility in healthy young children. Pediatr Neurol 2018; 86: 52–56.
- Molenaar HM, Zuidam JM, Selles RW, Stam HJ, Hovius SE. Age-specific reliability of two grip-strength dynamometers when used by children. J Bone Joint SurgAm 2008; 90: 1053–1059.
- Yang LJ, Anand P, Birch R. Limb preference in children with obstetric brachial plexus palsy. PediatrNeurol 2005; 33: 46–49.
- Bell-Krotoski J, Weinstein S, Weinstein C. Testing sensibility, including touch-pressure, two-point discrimination, point localization, and vibration. J Hand Ther 1993; 6: 114–123.
- Weinstein S. Fifty years of somatosensory research: from the Semmes-Weinstein monofilaments to the Weinstein Enhanced Sensory Test. J Hand Ther 1993; 6: 11–22.
- Mallet J. Obstetrical paralysis of the brachial plexus. II. Therapeutics. Treatment of sequelae. Priority for the treatment of the shoulder. Method for the expression of results. Rev Chir Orthop Reparatrice Appar Mot 1972; 58 Suppl 1: 166–168.
- Schulz J, Henderson SE, Sugden DA, Barnett AL. Structural validity of the Movement ABC-2 test: factor structure comparisons across three age groups. Res Dev Disabil 2011; 32: 1361–1369.
- Molenaar HM, Selles RW, Zuidam JM, Willemsen SP, Stam HJ, Hovius SE. Growth diagrams for grip strength in children. Clin Orthop Relat Res 2010; 468: 217–223.
- Petersen P, Petrick M, Connor H, Conklin D. Grip strength and hand dominance: challenging the 10% rule. Am J Occup Ther 1989; 43: 444–447.
- Strombeck C, Krumlinde-Sundholm L, Remahl S, Sejersen T. Long-term follow-up of children with obstetric brachial plexus palsy I: functional aspects. Dev Med Child Neurol 2007; 49: 198–203.
- Bechtol CO. Grip Test, The use of a dynamometer with adjustable handle spacings. J Bone Joint Surg 1954; 36-A: 820–824, 832.
- Zhang L, Zhang CG, Dong Z, Gu YD. Spinal nerve origins of the muscular branches of the radial nerve: an electrophysiological study. Neurosurgery 2012; 70: 1438–1441.
- Dumont CE, Forin V, Asfazadourian H, Romana C. Function of the upper limb after surgery for obstetric brachial plexus palsy. J Bone Joint Surg Br 2001; 83: 894–900.
- Anguelova GV, Rombouts S, van Dijk JG, Buur PF, Malessy MJA. Increased brain activation during motor imagery suggests central abnormality in Neonatal Brachial Plexus Palsy. Neurosci Res 2017; 123: 19–26.
- Anguelova GV, Malessy MJ, Buitenhuis SM, van Zwet EW, van Dijk JG. Impaired automatic arm movements in obstetric brachial plexus palsy suggest a central disorder. J Child Neurol 2016; 31: 1005–1009.
- Brown T, Cupido C, Scarfone H, Pape K, Galea V, McComas A. Developmental apraxia arising from neonatal brachial plexus palsy. Neurology 2000; 55: 24–30.
- Strombeck C, Remahl S, Krumlinde-Sundholm L, Sejersen T. Long-term follow-up of children with obstetric brachial plexus palsy II: neurophysiological aspects. DevMed Child Neurol 2007; 49: 204–209.
- Auer T, Pinter S, Kovacs N, Kalmar Z, Nagy F, Horvath RA, et al. Does obstetric brachial plexus injury influence speech dominance? Ann Neurol 2009; 65: 57–66.
- Siqueira MG, Heise CO, Pessa M, Zacariotto M, Martins RS. Long-term evaluation of hand function in children undergoing Oberlin and Oberlin-like procedures for reinnervation of the biceps muscle. Childs Nerv Syst 2020; 36: 3071–3076.
- van der Holst M, Geerdink Y, Aarts P, Steenbeek D, Pondaag W, Nelissen RG, et al. Hand-Use-at-Home Questionnaire: validity and reliability in children with neonatal brachial plexus palsy or unilateral cerebral palsy. Clin Rehabil 2018; 32: 1363–1373.