ORIGINAL ARTICLE
THE SWEDISH KING’S PARKINSON’S DISEASE PAIN SCALE: VALIDATION AND PAIN PREVALENCE IN PERSONS WITH MILD-MODERATE SEVERITY PARKINSON’S DISEASE
Conran JOSEPH, PhD1,2, Hanna JOHANSSON, PhD2,3, Breiffni LEAVY, PhD2,4 And Erika FRANZÉN, PhD2–4
From the 1Department of Health and Rehabilitation Sciences, Physiotherapy Division, Stellenbosch University, Cape Town, South Africa, 2Department of Neurobiology, Care Sciences and Society, Division of Physiotherapy, Karolinska Institutet, Huddinge, 3Medical Unit Occupational Therapy & Physiotherapy, Women’s Health and Allied Health Professionals Theme, Karolinska University Hospital, Stockholm and 4The Stockholm Sjukhem Foundation, Stockholm, Sweden
Objectives: To examine convergent and divergent validity of the King’s Parkinson’s disease Pain Scale – Swedish translated version, and to determine the prevalence of pain according to scale domains in persons with Parkinson’s disease.
Design: Cross-sectional, validation study.
Patients: Ninety-seven persons with Parkinson’s disease.
Methods: The pain scale was translated into Swedish by an accredited company, and permission was granted to use the resultant version. Participants completed the rater-administered The King’s Parkinson’s disease Pain Scale – Swedish version, the visual analogue scale (pain), Parkinson’s Disease Questionnaire (bodily discomfort subscale), MiniBESTest and Walk-12G. Spearman’s rank correlation coefficient was used to assess the strength of associations.
Results: The mean (standard deviation) age of participants was 71 (6.1) years, 63% were male, and 76% presented with mild disease severity. The mean (standard deviation) The King’s Parkinson’s disease Pain Scale – Swedish version score was 7.84 (12.8). A strong (r = 0.65) and moderate (r = 0.45) association was found between the newly-translated version and visual analogue scale (pain) and Parkinson’s Disease Questionnaire – bodily discomfort subscale, respectively. Weak associations were found between the newly translated version and divergent measures. Overall pain prevalence was 57%, with musculoskeletal pain being the most common, followed by chronic and radicular pain.
Conclusion: This study affirms aspects of validity of the Swedish King’s Parkinson’s disease Pain Scale. Most participants presented with 1 or more types of pain, highlighting the need for targeted interventions.
LAY ABSTRACT
When translating a scale from one language to another, it is important to assess the validity of the newly translated version. The aims of this study were to determine the validity of the newly translated Swedish version of the King’s Parkinson’s disease Pain Scale, and to quantify the number of persons with Parkinson’s disease who have reported pain using the translated version. The Swedish version of the pain scale was found to be closely associated with other pain scales, indicating measurement of the same behaviour. It was further found that 57% of persons with Parkinson’s disease in the study reported at least 1 type of pain, with most subjects experiencing musculoskeletal pain. In conclusion, the newly translated version of the pain scale is a valid assessment tool for pain in this population, and pain is very common in persons with Parkinson’s disease.
Key words: King’s Parkinson’s disease Pain Scale; pain assessment; Parkinson’s disease; pain prevalence.
Citation: J Rehabil Med 2023; 55: jrm9427. DOI: https://doi.org/10.2340/jrm.v55.9427
Copyright: © Published by Medical Journals Sweden, on behalf of the Foundation for Rehabilitation Information. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/)
Accepted: Apr 20, 2023; Published: Jun 12, 2023
Correspondence address: Conran Joseph, Division of Physiotherapy, Department of Health and Rehabilitation Sciences, Faculty of Medicine and Health Sciences, Francie van Zijl Drive, Parow, Cape Town, 8000, South Africa. E-mail: conran.joseph@ki.se; conran@sun.ac.za
Competing interests and funding: The authors have no conflicts of interest to declare.
In humans, the sequelae of Parkinson’s disease (PD) can be understood within 2 broad categories, namely motor (e.g. resting tremor, postural instability, bradykinesia) and non-motor (sleeping disorder, dementia, and pain) (1, 2). Pain is a common symptom in people with PD, manifesting in up to 85% of cases, and impacts an individuals’ functioning and quality of life (3, 4). However, the assessment of pain in this population has been challenging due to the lack of a standardized disease-specific measure capturing the complexity of pain origins.
Pain in PD can be classified as related or unrelated to the disease (5). Based on aetiology, Ford classifies pain in persons with PD into 5 categories: musculoskeletal pain, neuropathic radicular pain, dystonia-related pain, akathisia, and primary central parkinsonian pain (6). From these, pain can also be categorized as nociceptive, neuropathic, and miscellaneous, based on its underlying mechanism (6).
The King’s Parkinson’s disease Pain Scale (KPPS) is the first pain questionnaire specific for persons with PD, and has been found to be valid and reliable in its first design and development version (7). However, the original version was developed in English, and for it to be utilized in the Swedish healthcare context and among its Swedish-speaking citizens, it would need to be translated into the local language. Given that the translation process may alter the wording and phrasing of items, researchers should engage in a scientific process to ensure that the original construct under study, i.e. pain, is maintained (8).
Criterion and construct validity, 2 of the 4 aspects of validity assessment, is the ability to assess the construct/domain of behaviour under study, which is not directly observable (e.g. pain, mood, quality of life). In the case of translating a measure, apart from establishing the face and content validity, it should demonstrate both convergent and divergent validity (9). With convergent validity, the tested questionnaire should correlate highly with other measures testing the same construct; for example, the newly translated Swedish version of the KPPS and visual analogue scale (VAS) for pain. On the other hand, when assessing divergent validity, the tested questionnaire should demonstrate no or weak correlation with other measures testing a different construct; for example, the newly translated Swedish version of the KPPS and dynamic balance performance or perceived walking ability. Correlation matrices are then used to examine the expected patterns of associations between different measures of the same construct, and those between a questionnaire of a construct and other dissimilar constructs.
A high prevalence of pain has been reported in an earlier study conducted among persons with PD in Sweden (10). For this, a generic measure of pain and pain interference was used, providing limited insights into the nature and behaviour of pain specific to PD. Despite a high prevalence of pain being reported in Sweden, a low proportion of physiotherapists (< 30%), including primary care therapists, indicated that they had prioritized and treated pain in persons with PD (11). To mitigate the associated pain experience in persons with PD, it is necessary to better understand the origin and burden of pain in this population, which could be done with the use of a validated version of the Swedish Parkinson’s disease Pain Scale.
The aim of this study was to examine aspects of criterion and construct validity (convergent and divergent) of the Swedish translated version of the KPPS through hypotheses testing in persons with PD. For convergent validity, the study hypothesized a moderate-to-strong correlation between the total scores of the KPPS – Swedish version and Pain VAS and a moderate correlation between the KPPS – Swedish version and the PDQ-39 bodily discomfort subscale. In terms of divergent validity, a poor correlation between the total scores of the KPPS – Swedish version and Mini-BESTest, measuring balance performance, as well as between KPPS – Swedish version and Walk-12G, a self-rated outcome measure evaluating perceived walking ability, was expected. This study further aimed to describe the prevalence of pain in persons with mild-moderate PD according to the domains of the KPPS – Swedish version.
METHODS
Design and participants
A cross-sectional study design, founded on baseline assessments of participants who were enrolling in an implementation trial aimed at delivering a balance training regime for the assessment of neuroplastic changes, was used to determine the validity of the Swedish version of the KPPS, as well as the prevalence of pain in persons with mild-moderate PD. Ninety-seven persons with PD were included in this validation study, which, according to the COSMIN study design checklist, meets the requisite sample size for content/construct validation purposes (12).
Data collection procedure
Eligible participants were scheduled for baseline clinical assessments and interviews on a single day provided they meet the following inclusion criteria: (i) confirmed idiopathic PD diagnosis, according to the United Kingdom Parkinson’s Disease Society Brain Bank Criteria, of mild–moderate nature (H&Y II and III) by expert neurologists; (ii) had perceived balance dysfunction; (iii) able to walk indoors without a walking device; and (iv) provision of informed consent by eligible participants. Those with other neurological conditions, possibly influencing balance performance (e.g. stroke), as well as those with significant cognitive impairments, assessed using the Montreal Cognitive Assessment (MoCA) with a score lower than 21, were excluded. All participants were assessed by a physical therapist in the clinics during the “On” medication cycle 1–2 h after taking their anti-Parkinson medication. Throughout data collection, 3 interviewers were involved in the KPPS administration. All 3 interviewers were registered physiotherapists with vast experience in administering surveys and clinical tests associated with PD symptomatology. In terms of training conducted, the research team comprising 3 registered physiotherapists and 2 academics met to discuss the administration and scoring of the KPPS. The research team has more than 20 years’ experience (collectively) in PD assessment and management. “Dry runs” of questionnaire administration took place and all uncertainties were clarified.
The following data were collected from each eligible participant before enrolment in the clinical trial: (i) demographic factors; (ii) disease severity and functional ability (Hoehn and Yahr; Mini-BESTest); (iii) pain as measured with the KPPS, VAS pain scale, and the Bodily discomfort subscale of the PDQ-39; and (iv) disease-specific quality of life (single index PDQ-39 score 0–100) and perceived walking ability using the Walk-12G.
Instruments: outcome measures
King’s Parkinson’s disease Pain Scale: translation process. The original (English) version of the KPPS was translated by an accredited company (Corporate Translations, Inc. Hartford, Connecticut, USA) in accordance with current industry standards and United States of America (USA) guidance. The process of cross-cultural translation and validation was as follows: 2 bilingual translators produced individual Swedish translations of the KPPS, which was then subjected to discussion and consensus reaching to produce a harmonized forward translation. Subsequently, 1 independent back-translation was performed, which was then reconciled with the harmonized translation, as mentioned earlier. Three different stakeholders, namely a survey research expert, clinician, and on-site sponsor representative, reviewed the back-translated version and harmonized translation. Lastly, the harmonized translation was desktop published and proofread. Permission was granted to use the Swedish-translated version for further criterion and construct validation testing.
The KPPS has become internationally established and is an English-language, standardized, reliable and valid scale for evaluation of pain in idiopathic PD (7). The KPPS is a rater-interview-based scale, with the patient aided by the caretaker if necessary. The scale considers several pain dimensions, including location, severity, and frequency of pain across 7 domains, addressing pain syndromes identified in PD. The KPPS consists of 7 domains including 14 items; (i) musculoskeletal pain (domain 1); (ii) chronic/nociceptive pain (domain 2) with neuropathic pain included in domains 2 and 6 (discoloration; oedema/swelling). In addition, the scale includes fluctuation-related pain (domain 3), nocturnal pain (such as pain related to restless legs syndrome) (domain 4), orofacial pain (domain 5), and radicular pain (domain 7). Each item is scored by severity (0 – none to 3 – very severe) multiplied by frequency (0 – never to 4 – all the time), resulting in a sub-score of zero to 12 per item. The sum of sub-scores results in a possible total score ranging between zero and 168. This theoretical aggregation of combining severity and frequency has been used successfully in various widely validated scales (13).
Visual analogue scale. The VAS is a measurement instrument often used in epidemiological and clinical research to measure the intensity or frequency of various symptoms (14). This is, in many diagnostic groups, considered the gold standard for assessing subjective pain experience. The amount of pain that a patient/client can experience ranges from zero (none) to 10 (an extreme amount of pain). From the patient’s perspective, this spectrum appears continuous; their pain does not take discrete jumps, as a categorization of none, mild, moderate and severe would suggest. The simplest VAS is a straight horizontal line of fixed length, usually 100 mm. The ends are defined as the extreme limits of the parameter to be measured (symptom, pain, health), oriented from the left (best) to the right (worst).
Parkinson’s Disease Questionnaire-39. The PDQ-39 consists of 39 questions with 8 discrete scales: mobility (10 items), activities of daily living (6 items), emotional well-being (6 items), stigma (4 items), social support (3 items), cognition (4 items), communication (3 items), and bodily discomfort (3 items). Patients were asked to reflect on their health and general well-being, as well as to consider how often in the last month they have experienced certain events (e.g. difficulty walking 100 yards). Patients were further asked to indicate the frequency of each event by selecting 1 of 5 options on a Likert scale: never/occasionally/sometimes/often/always or cannot do at all. The sub-scores range from zero to 100, with a higher score reflecting greater impact of dimension on quality of life. Only the bodily discomfort dimensional score was used in the study, and was operationalized by means of 3 items, namely: (i) painful muscle cramps or spasms; (ii) joints and body aches and pains; and (iii) unpleasantly hot or cold experiences. The PDQ-39 is widely used worldwide, and it has been translated, culturally adapted, and validated into 13 different languages (15, 16).
Mini-BESTest. This clinical balance assessment tool is a shortened version of the Balance Evaluation Systems Test (BESTest). It aims to target and identify 6 different balance control systems, so that specific rehabilitation approaches can be designed for different balance deficits. The BESTest was shortened based on factor analysis to include dynamic balance only and to improve clinical utility.
The Mini-BESTest is a 14-item test scored on a 3-level ordinal scale. The Mini-BESTest assesses a unidimensional construct of dynamic balance through 4 of the 6 sections of the original BESTest, namely: (i) anticipatory postural adjustments; (ii) reactive postural control; (iii) sensory orientation; and (iv) dynamic gait. The total score ranges from zero to 28 points, with a higher score reflecting better dynamic balance control (17).
Walk-12G. The generic walking scale (Walk-12G) was adapted from the 12-item Multiple Sclerosis walking scale (MSWS-12v1), a self-administered questionnaire measuring walking impairments in multiple sclerosis (18). The MSWS-12 was subsequently modified, resulting in the Walk-12G, with the aim of being suitable for people with other neurological disorders, such as PD. The focus is on capturing patients’ perception of their difficulty with walking (in the past 2 weeks), resulting in a total score ranging from 0 to 42, where a higher score indicates greater difficulty (19).
Analyses
Descriptive statistics were used to describe participants, their key characteristics, as well as pain prevalence (any amount of pain experienced other than the absence of pain) and distribution according to the total and domain scores of the KPPS – Swedish version. Spearman’s rank correlation test was used to examine the strength of the relationship between the KPPS and the other pain (VAS), functional ability (Mini-BESTest), and health/wellbeing measures (PDQ-39 – bodily discomfort subscale; Walk-12G). The strength of the correlations was classified as; < 0.40 = poor, 0.41–0.60 = moderate, 0.61–0.80 = strong, and 0.81–1.00 = very strong (20). Inferential analyses were only carried out on cases where all main outcomes measures were completed, resulting in 88 participants.
Ethics
This study was approved by the Regional Ethical Review Board in Stockholm (2016/1264–31/4, 2017/1258–32 and 2017/2445–32). Participants received written and oral information about the study and all assessments, and were provided with written informed consent before the commencement of assessments.
RESULTS
Participant characteristics
Ninety-seven participants with mild to moderate PD were enrolled in this study (Table I), the majority of whom were male (n = 61). The mean age was 71 ± 61 years. In terms of disease severity, more than two-thirds of participants were categorized at mild disease stage (H & Y II) and one-quarter of the study sample reported using a mobility aid for outdoor ambulation.
Pain prevalence and summary statistics of outcome measures
The mean pain scores according to the VAS and KPPS – Swedish version were 18.80 (22.7) and 7.84 (12.8), respectively. The prevalence of pain according to the VAS and KPPS – Swedish version was 72% and 57%, respectively. The prevalence of pain as per the domains of the KPPS – Swedish version was: musculoskeletal pain (domain 1) at 37%; chronic pain (domain 2) at 20%; fluctuation-related pain (domain 3) at 8%; nocturnal pain (domain 4) at 19%; orofacial pain (domain 5) at 4%; discoloration: oedema/swelling (domain 6) at 9%; and radicular pain (domain 7) at 20%. Table II summarizes the central tendency and spread of the data.
Validity testing (criterion and construct)
Convergent validity. The Spearman’s correlation coefficient between KPPS and VAS was 0.65 (p < 0.001), indicating a strong relationship (Fig. 1). Also, VAS was compared with each individual intensity score of the 7 domains of the KPPS, where Spearman’s correlation coefficient ranged from 0.24 to 0.47 with a median coefficient of 0.32. The highest correlation was found with domain 1, capturing musculoskeletal pain (r = 0.47), while the lowest correlation was with domain 3 (r = 0.24), measuring fluctuation-related pain. Furthermore, a moderate correlation (r = 0.45; p < 0.001) was found between KPPS – Swedish version and the PDQ-39 Bodily Discomfort dimension (Fig. 2).
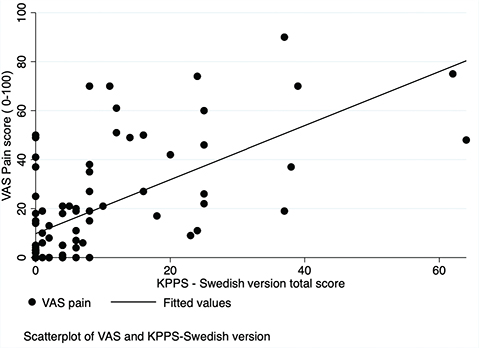
Fig. 1. Scatterplot: the King’s Parkinson’s Disease Pain Scale (KPPS) – Swedish version and visual analogue scale (VAS).
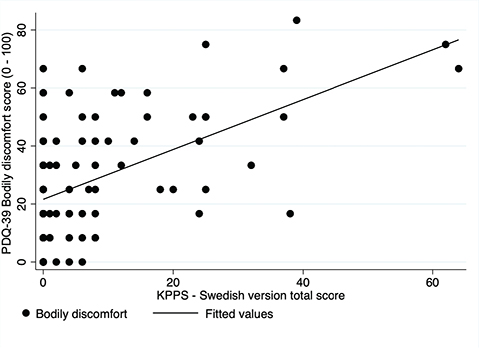
Fig. 2. Scatterplot: King’s Parkinson’s Disease Pain Scale (KPPS) – Swedish version and Parkinson’s Disease Questionnaire (PDQ)-39 (bodily discomfort).
Divergent validity of the KPPS Swedish version
Evidence of divergent validity was found between KPPS – Swedish version and Mini-BESTest (Fig. 3; r = –0.08; p = 0.44) and between KPPS – Swedish version and Walk-12G (Fig. 4; r = 0.21; p = 0.068).
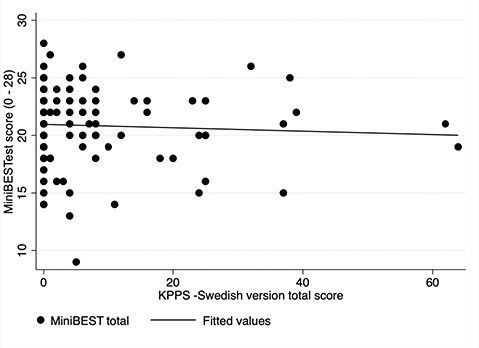
Fig. 3. Scatterplot: King’s Parkinson’s Disease Pain Scale (KPPS) – Swedish version and Mini-BESTest.
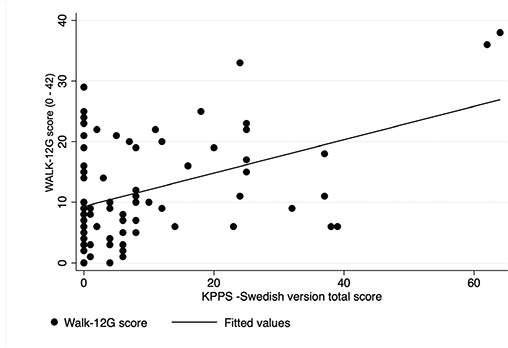
Fig. 4. Scatterplot: King’s Parkinson’s Disease Pain Scale (KPPS) – Swedish version and Walk-12G.
DISCUSSION
This study set out to establish aspects of criterion and construct validity of the Swedish-translated version of the rater-administered KPPS, and to determine the prevalence and severity of pain experienced according to the scale domains. The data underlying this study demonstrated adequate convergent and divergent validity, thus supporting the unidimensional construct (pain) being measured by the KPPS – Swedish version. In addition, the majority of participants (57%) experienced 1 or more types of pain, with most indicating musculoskeletal, chronic, or radicular type of pain. The latter findings provide therapeutic starting points for the management of pain in persons with PD.
Criterion and construct validation
A strong correlation was found between the KPPS – Swedish version and the VAS for pain in this study, providing evidence to support the underlying assessment of the same phenomenon, i.e. pain. The literature supports this strong correlation between the KPPS – Iranian version and VAS for pain, as well as for the Brief Pain Inventory (21). Another study by Behari et al. 2020 found a high correlation between the original English version of the KPPS and the Indian translated version (22). Furthermore, the total score of the KPPS (original and translated versions) correlates more highly with other pain measures (VAS) than the domains of the KPPS, as seen in the current study and others (7, 23). This is probably due to the low number of items per domain and subsequent spread of data within domains. Taken together, these findings support the convergent validity of rigorously translated versions of the KPPS for utility in PD populations across nations.
The current study further found confirmation of unidimensional pain assessment of the KPPS – Swedish version, as poor correlation was found between the latter measure and Mini-BESTest, measuring the construct dynamic gait, as well as between the KPPS – Swedish version and Walk-12G, which assesses perceived walking ability. In contrast, literature is available to support moderate correlations between the KPPS (original English and translated versions) and other non-pain measures, including Hoehn and Yahn disease severity, Hamilton Depression Rating Scale, Hamilton Anxiety Rating Scale, Pittsburg’s Sleep Quality Scale, and activities of daily living (23, 24). Authors allude to the pain experience in PD being related to the motor symptomatology of PD, which is integrally linked to the disease stages. For this reason, pain appears to be linked not only to motor symptoms, but also to non-motor symptoms, including mental health and sleep hygiene, which appear to affect those in the later stages of the disease progression (24, 25). This was not evident in the current study, as the included cohort was more homogenous with respect to disease severity.
Pain intensity and prevalence according to the King’s Parkinson’s disease Pain Scale
Pain intensity, according to the KPPS – Swedish version, was a mean (standard deviation; SD) of 7.84 (12.8) out of a possible 168, which is lower than reported for the KPPS – Chinese version (24) with a mean (SD) score of 41.2 (26.8), KPPS – Bulgarian version (26) with a mean score of 21.1 (17.3), and the KPPS – Indian version (22) with a mean score of 16.02 (10.57). The period-prevalence (i.e. pain experienced in the last month) of participants in the current study experiencing 1 or more types of pain was 57%. Data from India (using the KPPS – Indian version) and China (using the KPPS – Chinese version) suggest lower prevalence rates, at 52% and 44.5%, respectively, than found in the current study (22, 24). However, the period-prevalence in the current study was lower than in other regional studies: 79.6% in Bulgarian patients and 88.6% in Mexico City patients (23, 26). Reasons for the lower pain prevalence found in the current study could be attributed to the inclusion of majority mild disease severity (H & Y II) as well as the study sample comprising mainly males, as sources indicate a lower reporting of pain amongst men (27). In addition, since the current study was founded on a clinical trial, subjects with pain may be less interested in subscribing to balance training interventions, as it may provoke their pain.
In the current study, musculoskeletal, chronic, and radicular pain were found to be most prevalent. The leading cause of pain amongst persons with PD across various studies was found to be of musculoskeletal origin, with variation in the second and third most common types of pain. For example, the second and third most common types of pain in Bulgarian subjects with PD were nocturnal pain and fluctuation-related pain, respectively, while radicular and nocturnal pain were the second and third most common among Indian persons with PD, respectively (22, 26).
Musculoskeletal pain, as the most common pain, has its origins rooted in parkinsonian rigidity, stiffness, and immobility, and relieved by mobility (6). To mitigate this pain type, therapeutic options include both pharmacological and non-pharmacological interventions. Similarly, radicular pain is primarily attributed to nerve root entrapment, which is caused by poor posture due to rigidity and sedentariness. However, in extreme cases decompression surgery may be necessary to improve functioning and quality of life. This is, however, not without risks, considering the older age of persons with PD undergoing surgery. Unlike musculoskeletal and radicular pain origin, central pain may have an autonomic character and is presumed to be due to the disease itself. This type of pain appears to be difficult to manage (5, 6). In all, the early assessment of pain in persons with PD is important for diagnostic purposes, but also to promote early referral to rehabilitation professionals, such as physiotherapists, to assist in the management of the most common types of pain (musculoskeletal and radicular) more conservatively, as well as to reduce dependency on pharmacological treatments that are not curative in nature.
Methodological considerations and recommendations
Provided the validity assessment and prevalence estimates reported in this study, considering the following methodological considerations underlying the study are of importance. Although the sample size of this study was adequate for validation purposes, the range of persons with various stages of severity, mainly Hoehn and Yahn IV and V, was limited. Therefore, to ensure further validation and reliability, a follow-up study should include persons with PD from all disease severity stages, as well as a healthy control group, which may also provide sufficient variation for the assessment of known-group validity/discriminative validity, while intra- and inter-rater reliability studies could assist with the establishment of repeatability and internal consistency of the translated version. Furthermore, the selection of persons with PD with H&Y II and III only may have resulted in an under-estimation of pain prevalence as those with more advance stages of PD typically demonstrate greater pain intensity. To determine the burden of pain in persons with Parkinson’s disease, future longitudinal studies, including persons with PD of all severities, should be conducted to evaluate the evolution of pain, its nature, frequency, and intensity, as well as prescribed pain management interventions with the aim of assessing the evidence-based nature of current interventions.
In conclusion, this study affirms aspects of criterion and construct validity of the KPPS-Swedish translated version. In addition, even in this sample of people at predominantly mild disease stages, the majority of persons with PD experienced 1 or more types of pain, with the most frequent being musculoskeletal, central, and radicular in nature. Further refinement and subsequent implementation of the KPPS – Swedish version in clinical practice will help to determine the burden of disease-specific pain as a step towards optimized pain management in persons with PD.
ACKNOWLEDGEMENTS
The trial has been approved by the Regional Ethical Review Board in Stockholm 2016/1264–31/4, 2017/1258–32 and 2017/2445–32. We confirm that we have read the journal’s position on issues involved in ethical publication and affirm that all methods were carried out in accordance with relevant guidelines and regulations. Participants received written and oral information about the study and all assessments, as well as provided written informed consent before the commencement of assessments. All signed consent forms were filed in a locked cabinet.
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Funding was received from The Swedish Research Council and Center for Innovative Medicine (CIMED) – Karolinska Institutet and Region Stockholm, but had no influence on the design, data collection, analysis or interpretation of findings of the study.
REFERENCES
- Lees AJ, Hardy J, Revesz T. Parkinson’s disease. Lancet 2009 13; 373: 2055–2066. doi: 10.1016/S0140-6736(09)60492-X.
- Baig F, Lawton M, Rolinski M, Ruffmann C, Nithi K, Evetts SG, et al. Delineating nonmotor symptoms in early Parkinson’s disease and first-degree relatives. Mov Disord 2015; 30: 1759–1766. doi: 10.1002/mds.26281.
- Broen MP, Braaksma MM, Patijn J, Weber WE. Prevalence of pain in Parkinson’s disease: a systematic review using the modified QUADAS tool. Mov Disord 2012; 27: 480–484. doi: 10.1002/mds.24054.
- Rana AQ, Kabir A, Jesudasan M, Siddiqui I, Khondker S. Pain in Parkinson’s disease: analysis and literature review. Clin Neurol Neurosurg 2013; 115: 2313–2317. doi: 10.1016/j.clineuro.2013.08.022.
- Antonini A, Tinazzi M, Abbruzzese G, Berardelli A, Chaudhuri K, Defazio G, et al. Pain in Parkinson’s disease: facts and uncertainties. Eur J Neurol 2018; 25: 917-e69. doi: 10.1111/ene.13624.
- Ford B. Pain in Parkinson’s disease. Mov Disord 2010; 25: S98–S103. doi: 10.1002/mds.22716.
- Chaudhuri KR, Rizos A, Trenkwalder C, Rascol O, Pal S, Martino D, et al. King’s Parkinson’s disease pain scale, the first scale for pain in PD: an international validation. Mov Disord 2015; 30: 1623–1631. doi: 10.1002/mds.26270.
- Prakash V, Shah S, Hariohm K. Cross-cultural adaptation of patient-reported outcome measures: a solution or a problem? Ann Phys Rehabil Med 2019; 62: 174–177. doi: 10.1016/j.rehab.2019.01.006.
- Haertel E. Construct validity and criterion-referenced testing. Rev Educ Res 1985; 55: 23–46.
- Joseph C, Jonsson-Lecapre J, Wicksell R, Svenningsson P, Franzén E. Pain in persons with mild-moderate Parkinson’s disease: a cross-sectional study of pain severity and associated factors. Int J Rehabil Res 2019; 42: 371–376. doi: 10.1097/MRR.0000000000000373.
- Conradsson D, Leavy B, Hagströmer M, Nilsson MH, Franzén E. Physiotherapy for Parkinson’s disease in Sweden: provision, expertise, and multi-professional collaborations. Mov Disord Clin Pract 2017; 4: 843–851. doi: 10.1002%2Fmdc3.12525
- Mokkink LB, Terwee CB, Knol DL, Stratford PW, Alonso J, Patrick DL, et al. The COSMIN checklist for evaluating the methodological quality of studies on measurement properties: a clarification of its content. BMC Med Res Methodol 2010; 10: 1–8. doi: 10.1186/1471-2288-10-22
- Martinez-Martin P, Manuel Rojo-Abuin J, Rizos A, Rodriguez-Blazquez C, Trenkwalder C, Perkins L, et al. Distribution and impact on quality of life of the pain modalities assessed by the King’s Parkinson’s disease pain scale. Park Dis 2017; 3: 8. doi: 10.1038/s41531-017-0009-1.
- Paul-Dauphin A, Guillemin F, Virion JM, Briançon S. Bias and precision in visual analogue scales: a randomized controlled trial. Am J Epidemiol 1999; 150: 1117–1127. doi: 10.1093/oxfordjournals.aje.a009937.
- Peto V, Jenkinson C, Fitzpatrick R, Greenhall R. The development and validation of a short measure of functioning and well-being for individuals with Parkinson’s disease. Qual Life Res 1995; 4: 241–248. doi: 10.1007/BF02260863.
- Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N. The Parkinson’s Disease Questionnaire (PDQ-39): development and validation of a Parkinson’s disease summary index score. Age Ageing 1997; 26: 353–357. doi: 10.1093/ageing/26.5.353.
- Franchignoni F, Horak F, Godi M, Nardone A, Giordano A. Using psychometric techniques to improve the Balance Evaluation System’s Test: the mini-BESTest. J Rehabil Med 2010; 42: 323. doi: 10.2340/16501977-0537.
- Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ. Measuring the impact of MS on walking ability. Neurology 2003; 60: 31. doi: 10.1212/wnl.60.1.31.
- Leavy B, Löfgren N, Nilsson M, Franzén E. Patient-reported and performance-based measures of walking in mild–moderate Parkinson’s disease. Brain Behav 2018; 8: e01081. doi: 10.1002/brb3.1081.
- Riffenburgh RH. Statistics in medicine. San Diego: Academic Press; 2012.
- Taghizadeh G, Joghataei MT, Goudarzi S, Bakhsheshi M, Habibi SAH, Mehdizadeh M. King’s Parkinson’s disease pain scale cut-off points for detection of pain severity levels: a reliability and validity study. Neurosci Lett 2021; 745: 135620. doi: 10.1016/j.neulet.2020.135620.
- Behari M, Srivastava A, Achtani R, Nandal N, Dutta RB. Pain assessment in Indian Parkinson’s disease patients using King’s Parkinson’s Disease Pain Scale. Ann Indian Acad Neurol 2020; 23: 774. doi: 10.4103/aian.AIAN_449_20.
- Rodríguez-Violante M, Alvarado-Bolaños A, Cervantes-Arriaga A, Martinez-Martin P, Rizos A, Chaudhuri KR. Clinical determinants of Parkinson’s disease-associated pain using the King’s Parkinson’s Disease Pain Scale. Mov Disord Clin Pract 2017; 4: 545–551. doi: 10.1002/mdc3.12469.
- Gao L, Huang W, Cai L, Peng Y. Pain assessment in Chinese Parkinson’s disease patients using King’s Parkinson’s Disease Pain Scale. J Pain Res 2022; 15: 715–722. doi: 10.2147%2FJPR.S353249
- Tinazzi M. Pain and motor complications in Parkinson’s disease. J Neurol Neurosurg Psychiatry 2006; 77: 822–825. doi: 10.1136%2Fjnnp.2005.079053
- Stoyanova-Piroth G, Milanov I, Stambolieva K. Translation, adaptation and validation of the Bulgarian version of the King’s Parkinson’s Disease Pain Scale. BMC Neurol 2021; 21: 357. doi: 10.1186/s12883-021-02392-5
- Gao L, Yang Y, Cai L, Xiong Y. Gender differences in pain subtypes among Patients with Parkinson’s disease. J Integr Neurosci 2022; 21: 120. doi: 10.31083/j.jin2104120.