ORIGINAL ARTICLE
TWO-YEAR MORTALITY AND END-OF-LIFE DECISIONS AFTER TRAUMATIC SPINAL CORD INJURY: DATA FROM A LEVEL 1 TRAUMA CENTRE IN THE NETHERLANDS
Menco J. S. NIEMEYER, MD1, Felix PEUKER1, Said SADIQI, MD PhD2, Monika C. KERCKHOFFS, MD PhD3, R. Marijn HOUWERT, MD PhD1, Karlijn J. P. VAN WESSEM, MD PhD1, Marcel W. M. POST, PhD4,5 and Janneke M. STOLWIJK, MD PhD4
From the 1Department of Trauma Surgery, 2Department of Orthopedics, 3Department of Intensive Care Medicine, 4Centre of Excellence for Rehabilitation Medicine, De Hoogstraat Rehabilitation and University Medical Centre Utrecht (UMCU) Brain Centre, UMCU, Utrecht and 5University of Groningen, University Medical Centre Groningen, Centre for Rehabilitation, Groningen, The Netherlands
Objective: Literature shows high in-hospital mortality rates following end-of-life decisions in patients with traumatic spinal cord injury. This study investigated 2-year mortality and end-of-life decisions in patients with traumatic spinal cord injury.
Design: Explorative retrospective study in a Dutch level 1 trauma centre.
Patients: All consecutive patients between 2015 and 2020 with new traumatic spinal cord injury were selected from the trauma registry. Patients were excluded if myelopathy, cauda equina, or conus medullaris injury was absent or if they were referred to another level 1 trauma centre.
Methods: Mortality and end-of-life decisions (i.e. withdrawal and withholding of treatment, and euthanasia) within 2 years were analysed. Demographics, injury and clinical characteristics, and hospital treatment outcomes were compared with survivors. Motivations and critical morbidities concerning end-of-life decisions were assessed.
Results: The sample included 219 patients. Two-year mortality was 26% (n = 56), in-hospital mortality was 16%. The deceased were older, had more comorbidities and more severe injuries. end-of-life decisions concerned 42 patients (75%), mostly motivated by loss of independence or poor outcomes. Three patients received euthanasia (5%). The largest group with end-of-life decisions also sustained moderate-severe traumatic brain injuries (n = 11; 26%).
Conclusion: Most patients with traumatic spinal cord injury died following an end-of-life decision, with the largest group sustaining concomitant traumatic brain injuries. The incidence of euthanasia was low.
LAY ABSTRACT
Injuries to the spinal cord due to an accident, i.e. traumatic spinal cord injuries, are rare but have profound and life-altering consequences. Recent trends indicate that mostly elderly patients are affected. End-of-life decisions often arise when medical interventions have limited benefits or when injuries result in unbearable living conditions. Surprisingly, there is limited research on end-of-life decisions in patients with traumatic spinal cord injuries. This study examined the deaths of all patients with traumatic spinal cord injuries, and the frequency of end-of-life decisions in patients admitted to a Dutch trauma centre between 2015 and 2020. Among the 219 patients, a quarter died within 2 years, of which three-quarters died after an end-of-life decision. Euthanasia was performed in 3 cases. Furthermore, moderate-to-severe brain injuries were observed in one-third of the patients. This research emphasizes the need for further investigation into end-of-life decisions after traumatic spinal cord injuries, as it is crucial for informed decision-making and providing evidence-based end-of-life care.
Key words: brain injuries, traumatic; spinal cord injuries; end of life; euthanasia; medical futility; withholding treatment; withdrawal of treatment.
Citation: J Rehabil Med 2023; 55: jrm9584. DOI: https://doi.org/10.2340/jrm.v55.9584
Copyright: © Published by Medical Journals Sweden, on behalf of the Foundation for Rehabilitation Information. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Aug 22, 2023; Published: Nov 14, 2023
Correspondence address: Menco Niemeyer, University Medical Centre Utrecht, Department of Trauma Surgery, Heidelberglaan 100, NL-3584 CX Utrecht, The Netherlands. E-mail: m.j.s.niemeyer@umcutrecht.nl
Competing interests and funding: The authors have no conflicts of interest to declare.
In recent decades, notable progress has been observed in the outcomes, survival rates, and life expectancies of patients after traumatic spinal cord injury (TSCI) (1). These advancements are primarily attributed to the continuous development of acute care regimens, surgical interventions, and rehabilitation strategies (2–4). Survival of the acute phase after TSCI may initially appear to be a triumph, but it may also result in more patients with unfavourable long-term prospects. Consequently, this may give rise to more frequent and difficult choices for patients, their families, and healthcare providers concerning the suitability of commencing or continuing treatment, considering the projected quality of life. These deliberations can culminate in end-of-life decisions (ELDs), which present intricate ethical dilemmas involving the potential risk of either excessive overtreatment or inadvertently becoming a self-fulfilling prophecy in case of premature withdrawal of life-sustaining treatment.
Practices concerning ELDs vary greatly per region and country, and are influenced by factors such as religion, culture, age, and comorbidities (5). Because the decision over life, let alone one another’s life, is a weighty responsibility, ELDs legislature and terminology are a topic for debate worldwide (6). However, serious advancements have been made towards international consensus over end-of-life practices and terminology in the academic community (7).
The Netherlands is among the first governments in the world with a legal framework for ELDs (8). In the Netherlands, ELDs include non-treatment decisions, i.e. withholding or withdrawal of life-sustaining treatment, euthanasia and physician-assisted suicide (9). There is ambiguity regarding the moral difference between withholding and withdrawing life-sustaining therapies, as some clinicians are more hesitant to withdraw therapies compared with withholding treatment (10). Moreover, acute care specialists typically encounter non-treatment decisions in the acute phase, whereas euthanasia and physician-assisted suicide are typically committed well after injury (10).
A recent study in the Netherlands investigated in-hospital ELDs in patients with TSCI. The study found that level of injury, comorbidities, and age were associated with ELDs. The study also exemplified a lack of comparative literature and guidelines among Dutch hospitals and highlighted the challenges in decision-making for severe TSCI injuries (9). However, the data came from hospital discharge letters, and probably missed details regarding ELDs and only reported in-hospital mortality. It is possible that ELDs after new TSCI are also relevant after discharge and in other settings.
The aim of this research study was to investigate the 2-year mortality rate, the subsequent occurrence of ELDs, and to assess related clinical characteristics and motivations among patients with TSCI at a level-1 trauma centre in the Netherlands.
METHODS
The study is in accordance with Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (11).
Study design
This was a single-centre cohort study with an explorative retrospective assessment of all consecutive patients with spinal cord injuries from 1 January 2015 to 31 December 2020. Patients were selected from our regional trauma registry using the 2008 Abbreviated Injury Scale ≥ 1 in the spine region (12).
Setting
The study was conducted at the University Medical Centre Utrecht (UMCU). A level 1 trauma centre located in the central province of the Netherlands, a relatively small but highly populated urban area of 1,500 km2 and 1.3 million residents. The neurosurgery service area caters to 2.1 million inhabitants, with approximately 1,300 annual trauma admissions with full activation of a surgical trauma team (13).
The UMCU follows the Dutch end-of-life intensive care unit (ICU) guidelines, which require informed consent from the patient or family for decisions to withhold or withdraw life-sustaining treatment (14, 15). According to the hospital’s ICU end-of-life protocol, an ELD is considered when treatment is futile, unwanted, or disproportionate. In each case, all efforts are made to reach multidisciplinary consensus when deciding to stop recovery-oriented treatment. In addition to the responsible intensivist, the patient’s family (or the patient), involved consultants (especially the admitting specialty), and ICU nurses are important participants in the decision. If possible, at least 2 specialists are involved in the decision. The final decision to discontinue treatment always rests with the ICU treatment team (15).
In the Netherlands, euthanasia, and physician-assisted suicide, along with their respective due diligence requirements, are defined and governed by the Dutch Termination of Life on Request and Assisted Suicide Act (in Dutch: Wet toetsing levensbeëindiging) (16). Non-treatment decisions (i.e. withholding and withdrawing treatment) are addressed in the Dutch Medical Treatment Agreement Act (in Dutch: Wet op de geneeskundige behandelingsovereenkomst) (17).
Participants
Patients with spinal injuries were included when TSCI, traumatic cauda equina syndrome, or traumatic conus medullaris syndrome, hereafter all referred to as TSCI, was confirmed based on imaging and clinical assessment. Ambiguous cases were re-evaluated by an orthopaedic spine surgeon (author: SS) retrospectively for eligibility. Cases were excluded when myelopathy, cauda equina, or conus medullaris injury was absent or when referred to another level-1 trauma centre.
Outcome variables
The primary outcome was 2-year mortality and frequencies of ELDs within 2 years post-injury. Relevant information exceeding 2 years post-injury was omitted from analyses. Mortality analyses included comparison of patient, clinical and treatment outcome characteristics. Analyses of ELD included frequencies, and motivations for withdrawal or withholding of life-sustaining treatment, and euthanasia. Euthanasia was registered when specifically mentioned by the clinician as physician-assisted suicide or euthanasia following the due diligence process in accordance with and defined in the Dutch Euthanasia Law (16). Withdrawal of treatment was defined as an ELD followed by the discontinuation of life-sustaining devices or life-sustaining therapy. Withholding of treatment was defined as the withholding of any medical interventions that could otherwise prevent or significantly delay death. Decisions in patients who were withdrawn or withheld from further treatment following unsuccessful cardiopulmonary resuscitation, or while brain dead were not considered an ELD.
Secondary outcomes were variables associated with ELDs, collected through explorative data collection comprising quotes of interest from hospital records, associated injuries, such as concomitant traumatic brain injuries (TBI), and critical morbidities, defined as otherwise fatal or severely debilitating conditions, before ELDs.
Data sources and measurements
Data collection further encompassed patient demographics, injury characteristics (i.e. injury mechanism and severity, spine and spinal cord injury, traumatic cardiopulmonary resuscitation, Glasgow Coma Scores) provided by the Regional Trauma Care Network (Traumazorgnetwerk Midden-Nederland), the Dutch central region’s trauma admissions registry. Clinical parameters (i.e. ICU, hospital and rehabilitation hospital length of stay, stabilizing surgeries, neurological recovery) and case descriptions, ELDs, motivations for ELDs and follow-up on mortality were extracted from hospital records, which included discharge letters and documented correspondence with rehabilitation hospitals. Day of death on all admitted patients in UMCU is provided by the national death register on a real-time basis. Injury characteristics were classified according to the Abbreviated Injury Scale and Injury Severity Scale, spinal injuries to the AO Spine Classification Systems and the American Spinal Injury Association (ASIA) Impairment Scale (AIS) prior to discharge or death was used for neuro-logical outcome (12, 18–20). Comorbidities were defined and assessed in accordance with the Charlson Comorbidity Index (21).
Statistical analysis
Data were analysed using IBM SPSS Statistics, version 26.0.0.1 (Armonk, NY, USA). Variables in the deceased and survival groups were compared using the non-parametric Mann–Whitney U test in ordinal and continuous variables or Pearson’s χ2 test in dichotomous variables. Statistical significance was specified as p < 0.05. Continuous variables are presented as medians [interquartile range (IQR)] and categorical data are shown as absolute numbers (%).
Ethics approval
This study was approved by the UMCU institutional medical ethics review board. A waiver of consent for this study was approved and referenced under: WAG/mb/19/041369.
RESULTS
Between 1 January 2015, and 31 December 2020, a total of 219 patients were admitted with TSCI. The majority were male (66%) with a median [IQR] age of 58 [36–72] years. The largest group had had low-level falls (42%) followed by road traffic accidents (33%), of which the largest subgroup concerned cycling accidents (n = 32; 15%). Most injuries occurred in the cervical spine (68%), with distraction injuries (type B) being the most prevalent (45%). Additional demographic and injury characteristics are shown in Table I and Table SI.
| Variable | Total (n = 219) | Survived (n = 163) | Deceased (n = 56) | p-value |
| Sex (male), n (%) | 144 (66) | 104 (64) | 40 (71) | 0.16 |
| Injury mechanism, n (%) | 0.70 | |||
| Low-energetic falls | 91 (42) | 71 (44) | 20 (36) | |
| High-energetic falls | 43 (20) | 31 (19) | 12 (21) | |
| Road traffic accident | 73 (33) | 51 (31) | 22 (39) | |
| Other | 3 (1) | 3 (2) | n/a | |
| Unknown | 9 (4) | 7 (4) | 2 (4) | |
| Spine fracture typea, n (%) | 0.15 | |||
| Odontoidb | 16 (7) | 10 (61) | 6 (11) | |
| Compression (type A) | 37 (17) | 29 (18) | 8 (14) | |
| Distraction (type B) | 99 (45) | 78 (48) | 21 (38) | |
| Translation (type C) | 51 (23) | 33 (20) | 18 (32) | |
| Facet (type F) | 2 (1) | 2 (1) | n/a | |
| No fracture | 13 (6) | 11 (7) | 2 (4) | |
| Myelopathy level, n (%) | 0.56 | |||
| High cervical (C1–C4) | 82 (37) | 38 (23) | 14 (25) | |
| Low cervical (C5–C8) | 104 (47) | 80 (49) | 24 (43) | |
| Thoracolumbar | 43 (20) | 31 (19) | 12 (22) | |
| CMS/CES | 16 (8) | 12 | 4 (7) | |
| Pre-existing spondyloarthropathy, n (%) | 72 (33) | 55 (34) | 17 (30) | 0.75 |
| Traumatic CPRc, n (%) | 21 (11) | n/a | 21 (38) | < 0.01* |
| GCS < 8d, n (%) | 55 (25) | 44 (20) | 11 (20) | 0.71 |
| Spine max Abbreviated Injury Scale ≥ 3e, n (%) | 198 (90) | 150 (92) | 48 (86) | 0.29 |
| Head max Abbreviated Injury Scale ≥ 3e, n (%) | 39 (18) | 22 (14) | 17 (30) | < 0.01* |
| Severely injured (ISS ≥ 16), n (%) | 150 (69) | 99 (61) | 51 (91) | < 0.01* |
| Polytraumaf, n (%) | 66 (30) | 41 (25) | 25 (45) | 0.03* |
| Age, years, median [IQR] | 58 [36–72] | 53 [30–66] | 71 [56–79] | < 0.01* |
| Charlson Comorbidity Index, median [IQR] | 0 [0–1] | 0 [0–0] | 1 [0–2] | < 0.01* |
| ISS, median [IQR] | 20 [14–29] | 17 [10–26] | 28 [17–42] | < 0.01* |
| RTSg, median [IQR] | 7.84 [6.90–7.84] | 7.84 [7.84 8.84] | 5.81 [4.09 7.84] | 0.01* |
| aThe injury type corresponding with the spinal cord lesion is displayed according to the AO Spine classification system. bThe AO Spine classifications system identifies upper cervical fractures separately; atlanto-occipital fractures are displayed in total. cIncludes prehospital and the emergency room CPR. dTwenty percent of cases were missing compared with 2% in the surviving group; these patients remained unconsciousness or died quickly after admission, prohibiting history retrieval. eShown in frequencies of the Max Abbreviated Injury Scale ≥ 3 score per body region in a patient. One patient can have multiple injuries ≥ 3. fDefined as the Abbreviated Injury Scale ≥ 3 of 2 or more body regions. gDisplayed as the weighted sum of its components; values < 4 are associated with high mortality rates and must be transported to higher level trauma centres. | ||||
| SCI: spinal cord injury; CMS: conus medullaris syndrome; CES: cauda equina syndrome; CPR: cardio-pulmonary resuscitation; GCS: Glasgow Coma Scale; ISS: Injury Severity Score; IQR: interquartile range; RTS: Revised Trauma Score. *Statistical significance is defined as p < 0.05 | ||||
The median hospital length of stay was 10 [3–16] days. Nearly half of the patients were admitted to the ICU with a median length of stay of 0 [0–4] days (Table II). Most patients (72%) received spine stabilizing surgery. At hospital discharge, 36 patients (16%) had complete TSCI (AIS A), and the largest group (34%) was discharged with minor neurological deficits (AIS D). Seventeen percent of all patients showed full recovery at discharge (AIS E). Further clinical and outcome parameters are shown in Table II. Of the patients who survived, 8 (5%) were ventilator dependent in their (nursing) home setting. Futher clinical and outcome parameters are shown in Table II.
| Variable | Total (n = 219) | Survived (n = 163) | Deceased (n = 56) | p-value |
| Received stabilizing surgery, n (%) | 157 (72) | 128 (79) | 29 (52) | <0.01* |
| ICU admissions, n (%) | 104 (47) | 77 (47) | 27 (48) | 0.76 |
| ASIA Impairment Scalea, n (%) | <0.01* | |||
| AIS-A | 36 (16) | 18 (11) | 18 (32) | |
| AIS-B | 23 (11) | 11 (7) | 12 (21) | |
| AIS-C | 30 (14) | 22 (13) | 8 (14) | |
| AIS-D | 74 (34) | 69 (42) | 5 (9) | |
| AIS-E | 37 (17) | 36 (22) | 1 (2) | |
| Polytrauma/TBIb | 12 (5) | 7 (4) | 5 (9) | |
| Died before neurological examination | 7 (3) | n/a | 7 (13) | |
| Admissions to clinical rehabilitationc, n (%) | 95 (43) | 87 (56) | 10 (18) | <0.001* |
| ICU-LOS, days, median [IQR] | 0 [0–4] | 0 [0–4] | 0 [0–5] | 0.87 |
| Hospital-LOS, days, median [IQR] | 10 [3–16] | 12 [6–20] | 3 [2–7] | 0.01* |
| Rehabilitation-LOS, daysc, median [IQR] | 86 [46–130] | 85 [46–127] | 93 [51–149] | 0.43 |
| aThe neurological examination prior to discharge or death is displayed. bNeurological examination was not possible in these patients nor radiological estimation of the spinal cord injury. cConcerns only full admissions, no off-ward rehabilitation referrals. | ||||
| ICU: intensive care unit; ASIA: American Spinal Injury Association; TBI: traumatic brain injury; LOS: length of stay; IQR: interquartile range. *Statistical significance is defined as p < 0.05. | ||||
Mortality
The 2-year mortality rate was 26%, of which most patients (n = 35/56; 63%) died in hospital. Deceased patients were generally older (p < 0.01), had more comorbidities (p < 0.01), were more severely injured (Injury Severity Score; p < 0.01), sustained more moderate to severe TBI (p < 0.01), and had more severe AIS scores (p < 0.01) compared with survivors. There were no differences in height of myelopathy (Table II). Three patients (5%) died due to other causes (i.e. 1 died due to exsanguination, 1 due to severe sepsis, and 1 was declared brain dead). Eleven of the deceased patients were lost-to-follow up, their last known discharge locations are shown in Fig. 1.
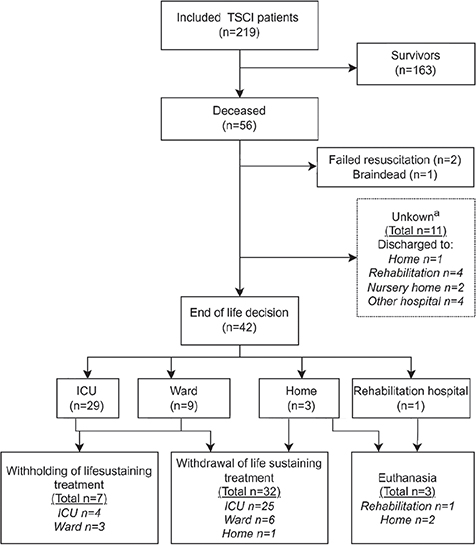
Fig. 1. Flowchart of mortality and end-of-life decisions. TSCI: traumatic spinal cord injury; ICU: intensive care unit. aOnly dates of death were retrieved from national governmental database; however, additional mortality; information was missing. The last-known discharge information is displayed.
End-of-life decisions
Most deaths in the study (n = 42/56; 75%) were associated with ELDs. Most ELDs occurred during hospital admission (n = 32/42, 76%), with a majority in the ICU (60%). One ELD (2%) was in a patient with readmission with pulmonary complications. A flowchart with details on ELD setting is shown in Fig. 1. The largest group among patients after an ELD had C1–C4 injuries (n = 17/42, 40%), followed by C5–C7 injuries (n = 14/42, 33%), and they predominantly had complete TSCI (AIS-A: n = 16/42; 38%) followed by motor complete TSCI (AIS-B; n = 8/42, 19%). In 9 cases (21%) the AIS was inconclusive due to severe polytrauma or sustained unconsciousness. Concomitant brain injuries were recorded in 15 patients (31%), i.e. severe TBI (n = 11, 26%) and brain hypoxia (n = 4, 12%). Severe TBI (n = 11, 26%), respiratory failure (n = 8, 19%) and severe pre-existent co-morbidities were the most prevalent critical morbidities associated with ELD.
More patients were withdrawn than withheld from life-sustaining treatment (respectively n = 32/42, 76% vs n = 7/42, 17%). One ELD in the home setting concerned withdrawal from mechanical home ventilation (Fig. 1). Three patients (7%) underwent euthanasia, on days 183, 268, and 722 post-injury, respectively. One euthanasia decision was motivated by loss of independence, the other 2 by loss of ventilatory function. All were performed in the home setting. Quotes from records on motivations and end-of-life conversations are shown in Table II.
In 23 patients (53%) the predicted poor outcome was mentioned as determinant for an ELD, whilst in 9 unconscious patients (21%) the loss of independence was decisive as accorded with the patient’s family. In 3 patients (7%), the clinical team predicted an unresponsive wakefulness syndrome before the ELD. Only 1 patient (4%) had a non-ICU advance directive before admission. None had advance directives specifically regarding neurological injuries. Fig. 2 and Table SIII provide further details on motivations and critical morbidities.
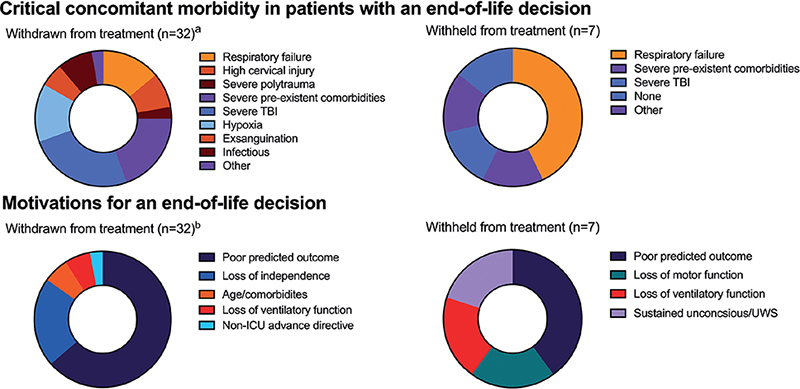
Fig. 2. Critical morbidities and motivations in patients with an end-of-life decision#. #Patients who received euthanasia were omitted due to low sample. Groups are arranged clockwise. ICU: intensive care unit; UWS: unresponsive wakefulness syndrome. aSix patients sustained multiple decisive critical morbidities (i.e. patients with, respectively, severe traumatic brain injury (TBI) and severe polytrauma, severe TBI and exsanguination, pneumonia and metastatic disease, respiratory failure, and severe comorbidities, and 2 patients with critical urosepsis with pre-existent tetraplegia). bIn 2 of the 3 patients in whom loss of independence was indicated potential referral to a nursing home was also decisive, and 1 of the 3 also mentioned loss of respiratory function.
DISCUSSION
This retrospective cohort study conducted at a Dutch level 1 trauma centre adds to the existing body of Dutch epidemiological data on TSCI. The study reveals a 2-year mortality rate of 26%, with the majority of deaths (75%) attributed to ELDs. Interestingly, only 3 cases of euthanasia were identified. Notably, results indicated an association between ELDs and TBI, suggesting an association between these injuries and predicted outcome.
To our knowledge, only 2 studies previously reported on ELD incidences in patients with TSCI (9, 22). A Dutch study by Osthertun et al. (9) reported on ELDs on a national scale with a slightly higher in-hospital mortality rate (19%) and slightly lower (63%) reported ELDs compared with the current study (16% and 75%, respectively). However, this study may have been limited by under-reporting. Patients were selected from multiple hospital registries based on International Classification of Diseases (ICD-9) codes. This selection criterion is notorious for diagnostic inaccuracies. In addition, they were limited to brief discharge papers with possible omissions regarding ELDs (23). The current study corrected for this by using the Abbreviated Injury Scale of the spine as selection tool, this could subsequently be verified with radiological and clinical characteristics. This scoring system is extensively validated and widely used in research and hospital benchmarking, where each individual injury is typically verified by both trained data managers and trauma surgeons (19). Furthermore, we have collected extensive data from hospital and ICU records spanning a 5-year period, thereby providing more information on ELDs and their clinical characteristics. A study conducted by Blex et al. (22) focused on TSCI within a German trauma centre. The study revealed a lower in-hospital mortality rate of 6% and a comparable ELD rate of up to 70%. It is presumed that this disparity in mortality rates could be attributed to the lower incidence of TBIs, as well as a smaller number of patients concomitant TBIs in Blex et al.’s study (22). However, the reason behind the differing incidence of concurrent TBIs between our population and Blex et al.’s study (22) remains uncertain. Moreover, the lack of a precise definition for an ELD and further details on injury severity characteristics limit the extent of our comparative analysis.
In the current study, the number of euthanasia cases identified was relatively low. This finding may be deemed lower than would be expected based on the prevailing perspective within the academic community regarding the Dutch approach to ELDs. This viewpoint is exemplified by a study conducted by Wade et al. which investigated the prevalence of prolonged disorder of consciousness. Notably, the Dutch data from their analysis was excluded, with the authors stating that “studies from the Netherlands consistently highlight the comparatively low prevalence, which can be attributed to their specific clinical practices” (24). Furthermore, to the best of our knowledge, there are no other studies available that specifically report on incidences of euthanasia, apart from the study by Osterthun et al. (9), which reported no cases of euthanasia. The existing literature indicates that patients are most susceptible to experiencing suicidal thoughts shortly after sustaining a TSCI, but this risk tends to decrease within the first 2 years post-injury (25). However, patients with spinal cord injury expressed reservations about making an informed decision within this time-frame (26). Nevertheless, another study by Waals et al. (27) presented 3 in-depth cases of euthanasia following TSCI, 2 of which expressed and persisted on their ELD within several days after hospital discharge. In Table III, the first euthanasia case (Euthanasia case 1) demonstrates the thorough due diligence process in the Dutch context, highlighting the careful attention given to each individual’s unique circumstances. This case serves as an example that when the due diligence process is conducted with meticulous consideration and expertise, and is governed by a stringent legal framework, it can ensure the provision of high-quality care for patients with severe and debilitating TSCI.
The findings of this study reveal a concomitant traumatic brain injury (TBI) rate of 38%, which is consistent with the range of 40–47% reported in the literature (28, 29). In addition, the study suggests a potential link between concomitant TBI and ELDs in patients with TSCI. Patients who experience both injuries demonstrate poorer functional outcomes during rehabilitation and a higher prevalence of complete TSCI (29, 30). These results underscore the importance of increased awareness and further investigation into this specific subgroup of patients, which may require distinct management approaches. Furthermore, the presence of severe TBI-induced unconsciousness can further complicate ELDs, as patients are unable to express their own wishes. Osterthun et al. reported that 68% of patients were unconscious when an ELD was made, a rate comparable to the 74% found in the current study (9). This observation suggests that unconsciousness plays a significant role in ELDs among patients with TSCI, thus highlighting the critical importance of advance directives. However, apart from 1 “no-ICU” order, none of the advance directives were formally registered in our sample.
Limited knowledge exists regarding the motivations behind the use of ELDs in patients with TSCI, with the exception of several case reports and surveys conducted among clinicians. A study by Ball et al. (31) examined the opinions of clinicians and identified 2 determinants for the use of ELDs in patients with TSCI: the absence of diaphragm function and the patient’s age. Moreover, clinicians in South African and Asian regions appeared to be more influenced by factors such as the family’s willingness to provide care and the availability of rehabilitation and long-term care, in comparison with Western respondents (31). The current study found that the main motivations for ELDs were the anticipated poor outcomes and the loss of independence. We suggest that these motivations are semantically comparable, as clinicians primarily observed and documented them as “poor outcomes,” which patients and/or their families may have perceived as “loss of independence”.
Study limitations
This study has several limitations. Firstly, it was a retrospective study with the limitations inherent to its design. This included 11 patients missing from mortality analysis. Although it was confirmed that these patients had died within the observed timeframe, it was not possible to determine whether their deaths were related to the use of ELDs. Consequently, the reported mortality and ELD rates in this study may underestimate the true figures. Furthermore, the process of categorizing complex considerations related to ELDs relied solely on an exploratory assessment of each case, based on information provided by the clinical team. This approach may have lacked comprehensive details and nuances. For example, the specific criteria used by the clinical teams to define “poor outcomes” were seldom described in detail in this study.
Secondly, this was a single-centre study, and therefore may not be generalizable to the entire Dutch population with TSCI. Even within the Netherlands, which is a relatively small, but densely populated, country, there are cultural and religious variations that may influence the experiences and perspectives of individuals with TSCI.
Thirdly, data collection and interpretation may have been subject to information bias, as the researchers noticed far more elaborate and detailed records on ELDs for younger and more severely injured patients compared with less injured and older patients. A prospective setup that includes interviews with clinicians, all patients with TSCI, and next-of-kin may address these confounders and provide additional insights.
Implications and future research
This study presents novel findings on mortality and rates of ELDs during the acute phase and is the first to observe these well after discharge. The significance of these findings is that the increasing incidence of TSCI disproportionately affects the elderly population in the Netherlands, who exhibit reduced resilience and diminished capacity for recovery (9, 32). Advances in acute care have contributed to a higher survival rate among elderly patients during the acute phase, thereby exposing them to potential ELDs more often, or subdugates patients to long-term morbidities that may necessitate the consideration of ELDs long after injury, including euthanasia. The high number of deaths following TSCI associated with ELDs, combined with the absence of comparative data, emphasizes the importance of evidence-based prognostication to facilitate informed and evidence-based ELDs.
To gain a deeper understanding of ELDs in the non-acute phase following injury, future studies should extend the total inclusion period. This extension will allow for the exploration of the relationship between ELDs and quality of life as well as functional outcomes. This could also provide more details on the Dutch practices concerning euthanasia in patients with TSCI. Furthermore, the results of this study may prove useful in development of a framework to elucidate decisive motivations. A future observational study with a prospective design should encompass a broad range of possible considerations relevant to clinicians, patients, and their families. In addition, future studies should incorporate functional outcomes and gather follow-up information several years post-injury to address existential questions. For instance, it would be valuable to enquire whether patients, in hindsight, would opt for an ICU admission again. Developing a prediction model would require comparing survivors and deceased individuals who had an ELD, considering clinical characteristics, functional outcomes, and patients’ perspectives on their outcomes.
In conclusion, this study, conducted at a Dutch level 1 trauma centre with a 2-year follow-up on mortality, provides important insights into ELDs in patients with TSCI. The findings reveal that the majority of TSCI-related deaths were preceded by ELDs. Factors such as old age, comorbidities, predicted loss of independence, and predicted poor outcomes were identified as decisive factors for ELDs in this population. The study also highlights the limited occurrence of euthanasia cases and the absence of advanced directives among the study participants. Furthermore, the results demonstrate an association between moderate-to-severe TBI and ELDs in patients with TSCI. These findings contribute to the understanding of ELDs in TSCI and emphasize the importance of informed decision-making in the management of these patients.
ACKNOWLEDGEMENTS
The authors thank Stasja Aspers from UMC Utrecht for retrieving medical records and data.
REFERENCES
- Bickenbach J, Officer A, Shakespeare T, von Groote P. A global picture of spinal cord injury. in International Perspectives on Spinal Cord Injury 2013. [Accessed 18-5-2023] Available from: https://apps.who.int/iris/rest/bitstreams/441640/retrieve.
- Niemeyer MJS, Lokerman RD, Sadiqi S, van Heijl M, Houwert RM, van Wessem KJP, et al. Epidemiology of traumatic spinal cord injury in the Netherlands: emergency medical service, hospital, and functional outcomes. Top Spinal Cord Inj Rehabil 2020; 26: 243–252.
- Devivo MJ. Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord 2012; 50: 365–372.
- Van Breugel JMM, Niemeyer MJS, Houwert RM, Groenwold RHH, Leenen LPH, Van Wessem KJP. Global changes in mortality rates in polytrauma patients admitted to the ICU – a systematic review. World J Emerg Surg 2020; 15: 1–13.
- Sprung L, Cohen SL, Sjokvist P, Lippert A, Phelan D. End-of-life practices in European. JAMA 2003; 290: 790–797.
- Deyaert J, Chambaere K, Cohen J, Roelands M, Deliens L. Labelling of end-of-life decisions by physicians. J Med Ethics 2014; 40: 505–507.
- Sprung CL, Truog RD, Curtis JR, Joynt GM, Baras M, Michalsen A, et al. Seeking worldwide professional consensus on the principles of end-of-life care for the critically Ill: The Am J Respir Crit Care Med 2014; 190: 855–866.
- van der Heide A, Onwuteaka-Philipsen BD, Rurup ML, Buiting HM, van Delden JJM, Hanssen-de Wolf JE, et al. End-of-life practices in the Netherlands under the Euthanasia Act. N Engl J Med 2007; 356: 1957–1965.
- Osterthun R, Van Asbeck FWA, Nijendijk JHB, Post MWM. In-hospital end-of-life decisions after new traumatic spinal cord injury in the Netherlands. Spinal Cord 2016; 54: 1025–1030.
- Sprung CL, Cohen SL, Sjokvist P, Lippert A, Phelan D. End-of-life practices in European. JAMA 2003; 290: 790–797.
- Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. PLoS Med 2007; 147: W163–W194.
- Champion HR, Panebianco NL, De Waele JJ, Kaplan LJ, Malbrain MLNG, Slaughter AL, et al. Abbreviated Injury Scale. Encyclopedia Intensive Care Med 2012; 1–5.
- Lansink KWW, Gunning AC, Leenen LPH. Cause of death and time of death distribution of trauma patients in a Level I trauma centre in the Netherlands. Eur J Trauma Emerg Surg 2013; 39: 375–383.
- Commissie Ethiek Nederlandse Vereniging voor IC, Fikkers BG, Gerritsen RTh, Meinders AJ, Olthuis GJ, Sijmons J, et al. [Dutch Guidelines for Palliative Care and Withdrawing Life-Prolonging Treatments in Adult ICU Patients 2021]. [accessed: 15-5-2023] Available from https://www.nvic.nl/wp-content/uploads/2022/01/Einde-leven-leidraad-2.0-februari-2021.pdf.
- Crane RF, Sikma MA, Hermens JAJ. Protocol: end of life care – Local protocol ICU UMC Utrecht 2022. Accessible through corresponding author.
- Regional Euthanasia Review Committee. Act on Review of Termination of Life on Request and Assisted Suicide (WTL), Criminal Code, and Funeral Act 2021 (in Dutch).
- Koninklijke Nederlandsche Maatschappij tot bevordering der Geneeskunst (KNMG). KNMG-richtlijn: Niet-aangaan of beëindiging van de geneeskundige behandelingsovereenkomst 2021. [accessed 17-5-2023] Available from: https://www.knmg.nl/advies-richtlijnen/dossiers/behandelingsovereenkomst-wgbo.htm.
- Kirshblum SC, Waring W, Biering-Sorensen F, Burns SP, Johansen M, Schmidt-Read M, et al. Reference for the 2011 revision of the International Standards for Neurological Classification of Spinal Cord Injury. J Spinal Cord Med 2011; 34: 547–554.
- Champion HR, Copes WS, Sacco WJ, Lawnick MM, Keast SL, Bain LW, et al. The major trauma outcome study: establishing national norms for trauma care. J Truma 1990; 30: 1356–1365.
- Schnake KJ, Schroeder GD, Vaccaro AR, Oner C. AO Spine Classification Systems (Subaxial, Thoracolumbar). J Orthop Trauma 2017; 31: 14–23.
- Charlson ME, Pompei P, Ales KL, Mackenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis 1987; 40: 373–383.
- Blex C, Kreutzträger M, Ludwig J, Nowak CP, Schwab JM, Lübstorf T, et al. Baseline predictors of in-hospital mortality after acute traumatic spinal cord injury: data from a level I trauma center. Sci Rep 2022; 1: 12.
- O’Malley KJ, Cook KF, Price MD, Wildes KR, Hurdle JF, Ashton CM. Measuring diagnoses: ICD code accuracy. Health Serv Res 2005; 40: 1620–1639.
- Wade DT. How many patients in a prolonged disorder of consciousness might need a best interests meeting about starting or continuing gastrostomy feeding? Clin Rehabil 2018; 32: 1551–1564.
- Kennedy P, Garmon-Jones L. Self-harm and suicide before and after spinal cord injury: a systematic review. Spinal Cord 2017; 55: 2–7.
- Tchajkova N, Ethans K, Smith SD. Inside the lived perspective of life after spinal cord injury: a qualitative study of the desire to live and not live, including with assisted dying. Spinal Cord 2021; 59: 485–492.
- Waals EMF, Post MWM, Peers K, Kiekens C. Experiences with euthanasia requests of persons with SCI in Belgium. Spinal Cord Ser Cases 2018; 4: 62.
- Budisin B, Bradbury CCLB, Sharma B, Hitzig SL, Mikulis D, Craven C, et al. Traumatic brain injury in spinal cord injury: frequency and risk factors. J Head Trauma Rehabil 2016; 31: 33–42.
- Hagen EM, Eide GE, Rekand T, Gilhus NE, Gronning M. Traumatic spinal cord injury and concomitant brain injury: a cohort study. Acta Neurol Scand 2010; 122: 51–57.
- Macciocchi SN, Bowman B, Coker J, Apple D, Leslie D. Effect of co-morbid traumatic brain injury on functional outcome of persons with spinal cord injuries. Am J Phys Med Rehabil 2004; 8: 22–26.
- Ball CG, Navsaria P, Kirkpatrick AW, Vercler C, Dixon E, Zink J, et al. The impact of country and culture on end-of-life care for injured patients: results from an international survey. J Trauma 2010; 69: 1323–1333.
- Nijendijk JHB, Post MWM, Van Asbeck FWA. Epidemiology of traumatic spinal cord injuries in the Netherlands in 2010. Spinal Cord 2014; 52: 258–263.