Implementation of perioperative FLOT compared to ECX/EOX chemotherapy regimens in resectable esophagogastric adenocarcinomas: an analysis of real-world data
DOI:
https://doi.org/10.2340/1651-226X.2024.35431Keywords:
Gastric cancer, esophageal cancer, FLOT, perioperative chemotherapy, Real-World Evidence, AdenocarcinomaAbstract
Background and purpose: Perioperative 5-FU, leucovorin, oxaliplatin, and docetaxel (FLOT) is recommended in resectable esophagogastric adenocarcinoma based on randomised trials. However, the effectiveness of FLOT in routine clinical practice remains unknown as randomised trials are subject to selection bias limiting their generalisability. The aim of this study was to evaluate the implementation of FLOT in real-world patients.
Methods: Retrospectively collected data were analysed in consecutive patients treated before or after the implementation of FLOT. The primary endpoint was complete pathological response (pCR) and secondary endpoints were margin-free resection (R0), overall survival (OS), relapse-free survival (RFS) tolerability of chemotherapy and surgical complications.
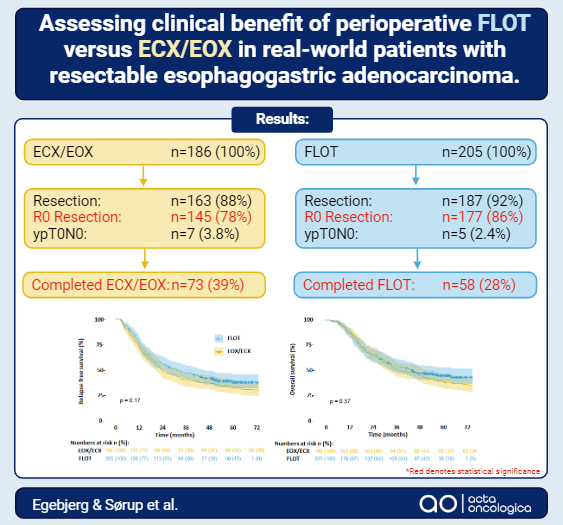
Results: Mean follow-up time for patients treated with FLOT (n = 205) was 37.7 versus 47.0 months for epirubicin, cis- or oxaliplatin, and capecitabine (ECX/EOX, n = 186). Surgical resection was performed in 88.0% versus 92.0%; pCR were observed in 3.8% versus 2.4%; and R0 resections were achieved in 78.0% versus 86.0% (p = 0.03) in the ECX/EOX and FLOT cohorts, respectively. Survival analysis indicated no significant difference in RFS (p = 0.17) or OS (p = 0.37) between the cohorts with a trend towards increased OS in performance status 0 (hazard ratio [HR] = 0.73, 95% confidence interval [CI]: 0.50–1.04). More patients treated with ECX/EOX completed chemotherapy (39% vs. 28%, p = 0.02). Febrile neutropenia was more common in the FLOT cohort (3.8% vs. 11%, p = 0.0086). 90-days mortality (1.2% vs. 0%) and frequency of anastomotic leakage (8% vs. 6%) were equal and low.
Interpretation: Patients receiving FLOT did not demonstrate improved pCR, RFS or OS. However, R0 rate was improved and patients in good PS trended towards improved OS.
Downloads
References
Arnold M, Ferlay J, Van Berge Henegouwen MI, Soerjomataram I. Global burden of oe-sophageal and gastric cancer by histology and subsite in 2018. Gut. 2020 Sep 1;69(9):1564–1571.
https://doi.org/10.1136/gutjnl-2020-321600 DOI: https://doi.org/10.1136/gutjnl-2020-321600
Then EO, Lopez M, Saleem S, Gayam V, Sunkara T, Culliford A, et al. Esophageal cancer: an updated surveillance epidemiology and end results database analysis. World J Oncol. 2020 Apr 1;11(2):55.
https://doi.org/10.14740/wjon1254 DOI: https://doi.org/10.14740/wjon1254
Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, et al. Pembrolizumab plus chemo-therapy versus chemotherapy alone for first-line treatment of advanced oe-sophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021 Aug 28;398(10302):759–771.
https://doi.org/10.1016/S0140-6736(21)01234-4 DOI: https://doi.org/10.1016/S0140-6736(21)01234-4
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376: 687-697.
https://doi.org/10.1016/S0140-6736(10)61121-X DOI: https://doi.org/10.1016/S0140-6736(10)61121-X
Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol [Internet]. 2015 Sep 1 [cited 2022 Nov 14];16(9):1090–1098. Available from: http://www.thelancet.com/article/S1470204515000406/fulltext DOI: https://doi.org/10.1016/S1470-2045(15)00040-6
Girling DJ, Bancewicz J, Clark PI, Smith DB, Donnelly RJ, Fayers PM, et al. Surgical re-section with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet. 2002 May 18;359(9319):1727–1733.
https://doi.org/10.1016/S0140-6736(02)08651-8 DOI: https://doi.org/10.1016/S0140-6736(02)08651-8
Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesopha-geal cancer. N Engl J Med. 2006 Jul 6;355(1):11–20. DOI: https://doi.org/10.1056/NEJMoa055531
https://doi.org/101056/NEJMoa055531
Al-Batran S-E, Homann N, Schmalenberg H, Kopp H-G, Haag GM, Luley KB, et al. Periope-rative chemotherapy with docetaxel, oxaliplatin, and fluorouracil/leucovorin (FLOT) versus epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) for resectable gastric or gastroesophageal junction (GEJ) adenocarcinoma (FLOT4-AIO): a multicenter, randomized phase 3 trial. J Clin Oncol. 2017 May 30;35(15_suppl):4004. DOI: https://doi.org/10.1200/JCO.2017.35.15_suppl.4004
https://doi.org/101200/JCO20173515_suppl4004
Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel ver-sus fluorouracil or capecitabine plus cisplatin and epirubicin for locally ad-vanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet [Internet]. 2019 May 11 [cited 2022 Jun 14];393(10184):1948–1957. Available from: http://www.thelancet.com/article/S0140673618325571/fulltext
Chen D. Real-world studies: bridging the gap between trial-assessed efficacy and routine care. J Biomed Res. 2022;36(3):147.
https://doi.org/10.7555/JBR.36.20220040 DOI: https://doi.org/10.7555/JBR.36.20220040
Al-Batran SE, Hofheinz RD, Pauligk C, Kopp HG, Haag GM, Luley KB, et al. Histopathologi-cal regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leuco-vorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, ran-domised phase 2/3 trial. Lancet Oncol. 2016 Dec 1;17(12):1697–1708. DOI: https://doi.org/10.1016/S1470-2045(16)30531-9
https://doi.org/10.1056/NEJMoa073149
Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med [Internet]. 2008 Jan 3;358(1):36–46. Available from: https://www.nejm.org/doi/10.1056/NEJMoa073149 DOI: https://doi.org/10.1056/NEJMoa073149
Becker K, Langer R, Reim D, Novotny A, Meyer Zum Buschenfelde C, Engel J, et al. Signifi-cance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: a summary of 480 cases. Ann Surg. 2011 May;253(5):934–939.
https://doi.org/10.1097/SLA.0b013e318216f449 DOI: https://doi.org/10.1097/SLA.0b013e318216f449
Langer R, Ott K, Feith M, Lordick F, Siewert JR, Becker K. Prognostic significance of histopathological tumor regression after neoadjuvant chemotherapy in esop-hageal adenocarcinomas. Mod Pathol. 2009 Oct 2;22(12):1555–1563.
https://doi.org/10.1038/modpathol.2009.123 DOI: https://doi.org/10.1038/modpathol.2009.123
Al-Batran S-E, Lorenzen S, Thuss-Patience PC, Homann N, Schenk M, Lindig U, et al. Sur-gical and pathological outcome, and pathological regression, in patients re-ceiving perioperative atezolizumab in combination with FLOT chemotherapy versus FLOT alone for resectable esophagogastric adenocarcinoma: interim re-sults from DANTE, a randomized, multicenter, phase IIb trial of the FLOT-AIO German Gastric Cancer Group and Swiss SAKK. J Clin Oncol. 2022 Jun 2;40(16_suppl):4003. DOI: https://doi.org/10.1200/JCO.2022.40.16_suppl.4003
https://doi.org/101200/JCO20224016_suppl4003
Larsen AC, Holländer C, Duval L, Schønnemann K, Achiam M, Pfeiffer P, et al. A nationwide retrospective study of perioperative chemotherapy for gastroesophageal adenocarcinoma: tolerability, outcome, and prognostic factors. Ann Surg On-col. 2014 Oct 28;22(5):1540–1547.
https://doi.org/10.1245/s10434-014-4127-2 DOI: https://doi.org/10.1245/s10434-014-4127-2
Siewert JR, Bottcher K, Stein HJ, Roder JD, Gastric G, Group CS, et al. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg. 1998 Oct;228(4):449.
https://doi.org/10.1097/00000658-199810000-00002 DOI: https://doi.org/10.1097/00000658-199810000-00002
Fischer B, Lassen U, Mortensen J, Larsen S, Loft A, Bertelsen A, et al. Preoperative staging of lung cancer with combined PET–CT. N Engl J Med. 2009 Jul 2;361(1):32–39. DOI: https://doi.org/10.1056/NEJMoa0900043
Additional Files
Published
How to Cite
Issue
Section
Categories
License
Copyright (c) 2023 Kristian Egebjerg, Tobias Sørup Andersen, Lene Bæksgaard, Rajendra Garbyal, Mette Siemsen, Michael Achiam, Paul Morten Mau-Sørensen

This work is licensed under a Creative Commons Attribution 4.0 International License.

