REVIEW ARTICLE
COST-EFFECTIVENESS OF PHYSICAL REHABILITATION AND CARE OF OLDER HOME-DWELLING PERSONS AFTER HIP FRACTURE: A SYSTEMATIC REVIEW AND NARRATIVE SYNTHESIS
Jonas Ammundsen IPSEN, PT, MScPT1,2, Lars T. PEDERSEN, MScPH1–3, Eva DARBORG, PhD4, Inge H. BRUUN, PhD1,2, Charlotte ABRAHAMSEN, PhD2,5 and Bjarke VIBERG, PhD, MD2,5
From the 1Department of Physical Therapy and Occupational Therapy, Lillebaelt Hospital, University Hospital of Southern Denmark, Kolding, 2Department of Regional Health Research, University of Southern Denmark, Odense, 3Department of Health Education, University College South Denmark, Esbjerg, 4Danish Centre for Health Economics, Department of Public Health, University of Southern Denmark, Odense and 5Department of Orthopaedic Surgery and Traumatology, Lillebaelt Hospital, University Hospital of Southern Denmark, Denmark, Kolding
Objective: To provide a systematic review of the literature and knowledge base of cost per quality-adjusted life year of physical rehabilitation and care of older persons after hip fracture.
Material and methods: A research librarian assisted in searching 9 databases (14 May to 27 May 2021), with exclusion of studies on cognitively impaired or institutionalized individuals. A stepwise selection process was conducted by 2 authors, study quality was assessed using Drummond et al.’s checklist, and comparison between different countries was assessed using Welte et al.’s checklist.
Results: Three studies were included, which employed 3 different interventions initiated at 3 different postoperative time-points. One high-quality study demonstrated that comprehensive geriatric assessment was cost-effective compared with coordinated care. The other 2 studies did not find the interventions studied to be cost-effective, and both studies were deemed to be of moderate quality.
Conclusion: The body of evidence on the cost-effectiveness of physical rehabilitation and care after hip fracture is limited and heterogeneous, with only 1 high-quality study. Thus, stakeholders perform decision-making with a limited knowledge base of the cost-effectiveness of physical rehabilitation and care. We recommend researchers to assess cost-per-QALY.
LAY ABSTRACT
Hip fractures have severe consequences for older persons and, after surgery, patients need physical rehabilitation and care to recover. Physical rehabilitation and care vary greatly in terms of effectiveness and cost. It is not known what kind of physical rehabilitation and care contribute most to health relative to their costs. This systematic review provides the first comprehensive description of the cost-effectiveness of physical rehabilitation and care of older persons after hip fracture. Nine databases were searched, and 3 economic evaluation studies were identified. One economic study identified comprehensive geriatric care as cost-effective compared with usual coordinated care. The other two studies consisting of an intervention of additional 10 weeks of physical rehabilitation initiated 4 months after discharge and an intervention physical rehabilitation and nutrient management proved not cost-effective compared to usual rehabilitation and care. In conclusion, the number of studies published in this field is very limited and further research is necessary.
Key words: systematic review; quality-adjusted life year; quality of life; cost-effectiveness; rehabilitation; care; costs; hip fracture.
Citation: J Rehabil Med 2022; 54: jrm00351. DOI: http://dx.doi.org/10.2340/jrm.v54.3421.
Copyright: © Published by Medical Journals Sweden, on behalf of the Foundation for Rehabilitation Information. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Oct 12, 2022; Epub ahead of print: Oct 31, 2022; Published: Nov 24, 2022
Correspondence address: Jonas Ammundsen Ipsen, Department of Physical Therapy and Occupational Therapy, Lillebaelt Hospital, University Hospital of Southern Denmark, Denmark, Kolding. E-mail: Jonas.Ammundsen.Ipsen@rsyd.dk
Competing interests and funding: The authors have no conflicts of interests to declare.
Hip fracture is the most common surgically treated trauma (1) and is associated with life-changing consequences for older home-dwelling persons, who experience reduced quality of life (QoL), physical function and mobility, as well as increased dependency on others (2, 3). After hip fracture, the most important goal for this patient group is to recover and regain independence (3). However, many patients do not regain their QoL or independence even a year after surgery (2, 4).
Physical rehabilitation and care are key interventions in facilitating recovery and improving QoL after hip fracture, and are routinely offered as individual or multifaceted interventions. The effectiveness of physical rehabilitation and care can vary greatly depending on the setting and content of the intervention (5–7).
A systematic review including 112 studies estimated the total world wide global cost per person in the first year after hip fracture as US$43,669. Physical rehabilitation and care was the second-largest driver of cost in this estimate, accounting for US$12,020 per person (8) and with 1.6 million expected yearly hip fractures world wide (Johnell O, Kanis JA (2006) An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17(12):1726–1733) hip fractures has a significant impact on healthcare resources consumption.
Prioritizing healthcare services based on cost-effectiveness is critical to the efficient utilization of resources (9). Thus, the cost-effectiveness of physical rehabilitation and care interventions is important in determining whether one intervention generates better, equal or worse outcomes than another, based on their relative consumption of resources. In addition to determining the relative impact physical rehabilitation and care interventions have on persons, cost-effectiveness estimates must also take into account the setting and content of each intervention. Economic evaluations are demanded by stakeholders and have a great potential for expanding the knowledge base, but, to our knowledge, no systematic reviews of studies assessing the economic dimensions of physical rehabilitation and care after hip fracture have been published. Therefore, the aim of this systematic review was to provide an overview of the literature and knowledge base of cost per quality-adjusted life year (QALY) of physical rehabilitation and care after hip fracture for persons aged 65 years and older.
METHODS
Protocol and registration
The systematic review was reported according to the updated Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (10) and conducted in adherence with the article series “How to prepare a systematic review on health economic evaluations for informing evidence-based healthcare decisions: a five-step approach” (11–14). The protocol was registered with PROSPERO (ID: CRD42021281984) and is accessible at https://www.crd.york.ac.uk/PROSPERO/
Design
A systematic review and meta-analysis were originally planned; however, the number of studies found was limited and heterogeneous regarding both when the interventions were initiated after surgery and the content of physical rehabilitation and care. Therefore, a narrative analysis was conducted instead. It was thus planned to conduct an exhaustive, comprehensive search for quantitative studies and to discuss the results in depth in order to elucidate the effect of the interventions (15).
Eligibility criteria
The research question was developed based on the population, intervention and outcome (PIO). The study populations was compromised of older home-dwelling persons (65 years or older). Interventions comprised physical rehabilitation and care programmes targeting improvement in the person’s physical functioning after hip fracture, which were mono- or multi-faceted, such as, but not limited to, physiotherapy, exercise and care interventions targeted improvement of the persons level of physical function after hip fracture (16, 17). The outcome measured was cost per quality-adjusted life year (QALY) in studies conducted in healthcare systems utilizing a single payer healthcare system comparable to those used in the Nordic countries (17, 18). Studies assessing interventions that targeted older persons with severe cognitive impairments, such as progressed dementia, or persons who were permanently institutionalized were excluded.
Information sources
Nine databases were selected based on their content descriptions at the University of Southern Denmark Library: MEDLINE, Embase, CINAHL, Cochrane Library, Scopus, the Health Technology Assessment (HTA) database of the Centre for Review and Dissemination, International HTA database, EconLit, and Academic Search Premier. All databases were deemed relevant by all authors and were searched from the date of inception.
Search strategy
Keywords were identified, assessed and arranged according to the PIO model. The search strategy was adapted to each database to account for differences in MeSH terms, indexation and matrix. All authors approved the keywords for each database. Grey literature in conference abstracts was searched. The search strategies are shown in Appendix S1.
A single author (JAI) performed all searches, during the period 14–27 May 2021.
Study selection
Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org, and a stepwise study selection process was conducted. Duplicates were removed, and 2 authors (JAI and LTPE) independently screened the remaining studies’ titles and abstracts. Next, both authors (JAI and LTPE) independently performed full-text screenings for final inclusion. In both steps (screening of title and abstract and full text), disagreements were resolved by consensus, which occasionally involved all authors (JAI, LTPE, ED, IHB, CA and BV).
Data extraction
A single author (JAI) completed a data extraction form, based on the form developed by Wijnenn et al. (14), which was subsequently verified by all authors. The form comprised 13 items relating to general study characteristics and 18 items relating to study methods and outcomes. The completed data extraction forms are available in Appendix S2.
The following data were extracted: first author, year of publication, year of trial, funding source, competing interests, publication type, setting, person characteristics, intervention type, control intervention, study eligibility criteria, study perspective, type of economic evaluation, analytical method, time-frame, discount rates for costs and effects, inflation rate, type and category of costs, data source of resource use, methods for identifying resource use, assumptions for measurement of resources, costs reported or converted currency, data source of effects, methods of measuring effects, methods of valuation of effects, effects, incremental cost-effectiveness ratio (ICER), analyses of uncertainty (e.g. sensitivity analyses), outcome(s) of sensitivity analyses and authors’ conclusions.
Disagreements were resolved through discussion and consensus between all authors.
Quality assessment
Quality assessment was performed using the commonly used checklist developed by Drummond et al., which was designed to appraise the quality of economic evaluations (9). The checklist was formatted as a table, with 1 axis showing each checklist criterion and the other axis presenting each economic evaluation, as suggested by Watts et al. (19). Each criterion was assessed as “Yes”, “No” or “Can’t tell”. The criteria for “Yes” are described in Appendix S3. Two authors (JAI and LTPE) independently assessed the studies and subsequently compared their findings. Disagreements were resolved by discussion between the 2 authors, and unresolved disagreements were discussed with an experienced health economist (EUD).
Transferability of studies
Welte et al.’s decision chart was used to assess the transferability of the study findings (20). The decision chart is practical in use and consists of 3 general knockout criteria and 14 specific criteria (14, 21, 20). To meet the first and second general criteria, the physical rehabilitation and care intervention and the comparator must be compatible with the decision country. To meet the third general criterion, the study must be of acceptable methodological quality, which was appraised by applying Drummond et al.’s checklist (20). The specific criteria assess relevance on a 4-point scale, ranging from “very high” to “very low” (20). Correspondence must be deemed “very high” or “high” to assume an unbiased cost-effectiveness ratio (CER) (20). As Welte et al.’s (21) decision chart requires a comparison between 2 countries, we pragmatically chose one Nordic country (Denmark) as reference country to compare study countries against. The assessment of transferability was conducted by 1 author (JAI), who conferred with an experienced health economist (ED). Disagreements were resolved by discussion.
Data synthesis
A narrative synthesis summarizing and interpreting the findings of the individual studies was conducted. To compare costs from studies completed in different years and currencies, the reported currency was converted to euros using the mean conversion rate for the trial completion year, based on historical conversion rates (22). Furthermore, costs were forward discounted from the trial completion year to 2021 using the national discount rate from Denmark of 3.5% and the equation P = Fn/1+R (P = present value; F=future value; n=number of years; R=interest rate) (9, 23) Table 3.
RESULTS
Study selection
The search located 1,493 studies, of which 502 duplicates were removed. After title and abstract screening, 953 studies were excluded, and, after full-text screening, 35 studies were excluded. Three studies remained and were included in this review. Two trial protocols currently recruiting were identified (24, 25), although as no results were available at the time of data extraction, these studies were not included. The study selection process and reasons for exclusion are shown in Fig. 1.
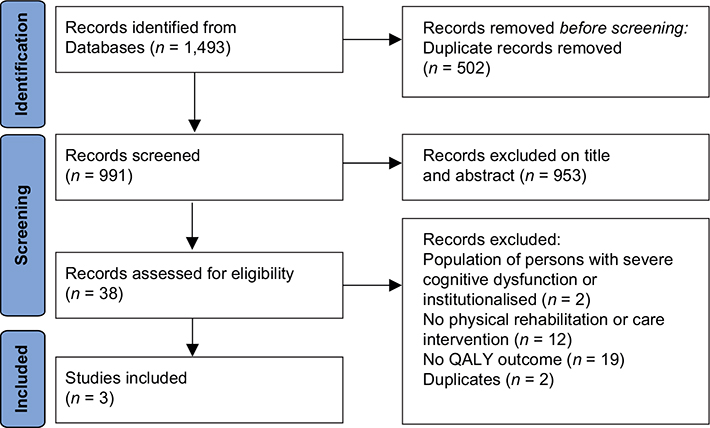
Fig. 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart. QALY: quality-adjusted life years.
Study characteristics
The 3 included studies were trials that applied a healthcare perspective encompassing the use of physical rehabilitation and care services in the primary and secondary sectors (26–28). The studies displayed heterogeneity in how costs were collected, valued and in QoL preference weights used (26–28). Two studies were based on trials completed in 2010 (26, 28) and 1 study was based on a trial completed in 2014 (27). One study was conducted in Australia (26) while 2 were conducted in Norway (27, 28). The interventions consisted of different types of physical rehabilitation and care, and were initiated at different postoperative time-points. The study characteristics are shown in Table I.
Milte et al. (26) assessed a 10-week individualized nutrition and exercise intervention initiated shortly after discharge after hip surgery. QoL outcomes were measured using the 5-dimension assessment of quality of life instrument (AQoL-4D) with preference weights for the general Australian population. Data collection was carried out weekly by trial staff. The questionnaire was used in combination with registry data encompassing the use of medical and pharmaceutical benefit schemes. The study’s time-frame was 6 months. Costs were adjusted to a 2010 consumer price index (trial year) and valued to accepted unit costs from the Australian National Hospital Cost Data Collection and cost of visits from allied healt professionals were taken from rebates specified by Department of Veterans Affairs.
Taraldsen et al. (27) assessed the outcomes of a 10-week, late-phase exercise programme initiated 4 months after discharge after hip surgery. QoL outcomes were measured using the EQ-5D-3L with English preference weights. Administrative registers, municipal person records and the Norwegian Directorate of Health were used to collect data on the use of healthcare services. Valuation of costs was based on fee-for-service information in Norwegian kroner (NOK) and reported in 2012 euros using the mean exchange rate from 2012. The study’s time-frame was 8 months.
Prestmo et al. (28) assessed the outcomes of a comprehensive geriatric assessment (CGA) at a geriatric hospital ward compared with usual care at an orthopaedic ward. QoL was measured using the EQ-5D-3L with English preference weights. Data on the use of healthcare services was obtained through administrative systems, municipal patient records, the Norwegian Patient Register and the Norwegian Health Economics Administration. Costs were valued using published costs or local experts and municipal websites in NOK and presented in 2010 euros based on the mean exchange rate from 2010. The time-frame of the study was 12 months.
Quality assessment
The study by Prestmo et al. (28) was determined to be of high quality, while the studies by Taraldsen et al. (27) and Milte et al. (26) were of moderate quality.
None of the studies achieved “Yes” ratings for all criteria, as they did not account for different time-frames or include all costs relevant to the healthcare perspective. Milte et al. (26) and Prestmo et al. (28) disclosed differential timing, though a comparison was deemed unfeasible due to their respective time-frames of 6 and 12 months. Taraldsen et al. (27) did not disclose their reasons for not adjusting for differential timing. The studies were heterogeneous in the costs included in the healthcare sector perspective, as, for instance, only 1 study, by Milte et al. (26), included use of medication in calculation of costs.
The studies’ included costs are detailed in Appendix S4.
Milte et al.’s study (26) was assigned ratings of “No” for 3 additional criteria. First, the study had an insufficient description of the comparator. Without knowledge of the contents and settings of usual physical rehabilitation and care in Australia, it was not possible to assess the comparative intervention. The second “No” was assigned for reporting an ICER estimate based on a minor statically insignificant difference in effect, which was inappropriate. The third “No” was due to the discussion, which did not reflect these concerns regarding the ICER estimate.
Taraldsen et al.’s study (27) was assigned “No” ratings on 2 additional criteria. First, the ICER was estimated and reported based on a small statistically insignificant difference in effect. Secondly, there was no reporting of an ICER plane or cost-acceptability curve, and the cause for not reporting an ICER plane was undisclosed, thus making the interpretation less transparent to the reader.
Prestmo et al.’s study (28) received “Yes” ratings for the remaining criteria.
The quality assessment of the 3 studies is shown in Table II.
Transferability
Milte et al. (26) fulfilled the first and third general knockout criteria. However, the second criterion was not fulfilled, as the description of usual physical rehabilitation and care was too general to adequately assess the content and setting of the comparator. Correspondence in practice variation was deemed “low”, as the mean length of stay of 16 days was considerably longer than usual practice in Nordic countries (26, 29). In addition, correspondence was “low” in 3 specific criteria. First, the inclusion of weekly social visits with the control group and the longer length of stay did not correspond well to procedures in Nordic countries. Secondly, the lack of a description of usual physical rehabilitation and care made direct comparisons between countries impossible. Thirdly, it is unknown how Australian QoL preferences compare with a Nordic population. As Danish and English QoL preferences do not equate, we cannot assume high correspondence between Australian and Nordic populations (30). Thus, the ICER estimate was considered biased.
Taraldsen et al. (27) met all 3 knockout criteria, and the correspondence between Norway and Nordic countries was deemed “high” (27). The healthcare perspective was narrower than recommended, although it is the most commonly used perspective in western countries (31). The ICER estimate was thus rated as unbiased.
Prestmo et al. (28) fulfilled the 3 general knockout criteria, and the correspondence between Norway and Nordic countries was deemed high. As the healthcare sector perspective was narrow, but the most commonly used, the ICER estimate was rated as unbiased (31).
The completed transferability decision charts are shown in Appendix S5.
Findings
Milte et al. (26) detected a difference in QALY gain of 0.02 (95% confidence interval (95% CI) –0.027, 0.059; intervention group 0.155 vs control group 0.139) (26), but the difference was not statistically significant. The mean total cost difference was €206.39 (95% CI –2,928.98, 3,468.72; intervention group €21,551.86 vs control group €21,268.93). Assuming the difference between groups was a true difference, the incremental cost per QALY was estimated as €13,471.14.
Taraldsen et al. (27) reported no difference in QALY gain between the groups (intervention group median 0.73 vs control group median 0.73) (27). The mean total cost difference was €51, 3 (95% CI –6.82, 6.75; intervention group €26,219 vs control group €25,976).
Prestmo et al. (28) demonstrated a statistically significant difference in QALY gain of 0.09 (95% CI 0.02, 0.16; intervention group mean 0.52 vs control group mean 0.45) (28). The total cost difference was –€3,528.00 (95% CI 2928.98, 3468.72; intervention group €37,213.52 vs control group €40,743.44). The incremental cost per QALY was –€49,145.53.
A summary of the studies’ findings is shown in Table III.
DISCUSSION
This systematic review presents the findings of 3 primary studies assessing different physical rehabilitation and care interventions compared with usual physical rehabilitation and care after hip fracture (26–28). Two of the studies showed that the interventions were not cost-effective, while the third study found the intervention to be cost-effective. Prior to this study PROSPERO (ID: CRD42021281984), the protocol was registered in Open Science Framework and remained unchanged during the review, except for the omission of a meta-analysis due to heterogeneity between studies.
The narrative synthesis revealed pronounced heterogeneity between studies, which is similar to a previous systematic review assessing the global cost of fragility hip fractures.which reported significant heterogeneity between studies affecting the credibility and accuracy of the results (31).
Prestmo et al. (28) demonstrated that CGA, including physical rehabilitation and care at a geriatric ward was more effective and less costly compared with usual care at an orthopaedic ward. In contrast, a Swedish study by Lofgren et al. (32), comparing coordinated rehabilitation and care at a geriatric ward with usual rehabilitation and care at an orthopaedic ward for hip fracture patients detected no difference between programmes in QoL. The difference between these 2 studies in the effect on QoL might be explained by differences in interventions (28, 32). CGA appears to be more comprehensive than coordinated rehabilitation; however, the descriptions were vague (28, 33). An additional explanation might be found in population differences, as Lofgren et al. (32) included persons living in nursing homes. Milte et al. (26) and Taraldsen et al. (27) did not find 2 different physical rehabilitation and care interventions to be cost-effective compared with usual physical rehabilitation and care in the primary sector. This may indicate that the content and scope of physical rehabilitation and care are important factors in improving persons’ QoL.
None of the included studies found their interventions to be more resource-demanding than usual physical rehabilitation and care (26–28). In 2 of the studies, this was probably due to fewer persons in the intervention group being admitted to nursing homes (27, 28). If nursing home admissions remain lower in the long term it might have implications for the cost-effectiveness ratio. This is potentially supported by an Australian study by Cameron et al. (34), which identified accelerated rehabilitation, including components of CGA, early mobilization and discharge programmes as less costly and as effective at recovering patients’ level of function as conventional rehabilitation. However as Prestmo et al. (28) followed persons for only 12 months and Taraldsen et al. for 8 months, it was not impossible to assess the long-term implications of the interventions (26–28). Thus, this should be assessed in future studies with a longer follow-up period, which, if feasible, are powered to the high mortality and drop-out rate of frail older persons.
Two of the included studies, by Taraldsen et al. (27) and Prestmo et al. (28), were conducted in a healthcare system organized in a primary sector (municipalities) and a secondary sector (hospital). In the study by Taraldsen et al. (27) the intervention imposed an increased and decreased use of municipal rehabilitation. In the study by Prestmo et al. (28) the intervention increased hospital cost and decreased the use of municipal care. Thus, in both studies the stakeholders paying the intervention were not the ones receiving the benefits. Based on the limited number of studies available, it was not possible to assess the significance of this potential barrier for implementation of new and more effective physical rehabilitation and care interventions.
Applying a narrow healthcare sector perspective in cost-effectiveness studies increases the risk of underestimating true resource use (9, 35). The 3 studies in this review included different costs in their assessments using the healthcare sector perspective (26–28). For example, Milte et al. (26) included the cost of social visits to the control group, while Taraldsen et al. (27) included the cost of psychiatric care in hospital, and Prestmo et al. (28) included the cost of hospital stays post-discharge. This indicates an overly narrow perspective of the minimal requirements of the healthcare sector. In contrast, the societal perspective is more feasible in older persons after hip fracture, as it includes the costs of informal care. Informal caregivers have been estimated to deliver a mean of 39.5 h of care per week in the first 6 months after hip fracture, and 36% of informal caregivers report a high perceived burden of care (36, 37).
Strengths and limitations
A strength of this systematic review was the very broad search performed in cooperation with a research librarian (13). To further exhaust the search, reference lists and grey literature were searched, though no additional relevant studies were identified. An additional strength was the study selection process, which was carried out independently by 2 researchers. Furthermore, study quality was assessed using a well-established checklist developed by Drummonds et al. (9), and 2 reviewers performed the assessment independently (19, 38).
Healthcare reimbursement schemes and the content of usual physical rehabilitation and care can bias or prevent credible comparisons of outcomes and costs between countries. Thus, the current review systematically assessed the transferability of study findings to a Nordic context using the Welte decision chart (20). This was carried out by a single author, and to reduce the risk of biased assessment, an experienced health economist advised in this process. A second assessor would have reduced the risk of assessor influence; however, it is not considered likely that a second accessor would have altered the assessment of transferability.
CONCLUSION
The evidence base of the cost-effectiveness of various physical rehabilitation and care interventions after hip fracture is limited and heterogeneous. Only 1 of 3 interventions was shown to be cost-effective. The studies used the same healthcare sector perspective, but did not include all relevant costs, and the interventions differed in content and were initiated at different postoperative time-points. This prevented pooled effect size estimates and clear recommendations for physical rehabilitation and care of older home-dwelling persons after hip fracture. Based on the findings of this systematic review, future economic evaluations should employ broader perspectives and a plan for longer follow-up to capture the long-term implications of physical rehabilitation and care. The inclusion of only 3 economic evaluations underscores the need for more economic research studies to support healthcare decision-making and prioritization, and highlights a gap in the current knowledge base.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge funding provided by the research council at Lillebaelt Hospital, the Association of Danish Physiotherapists, and the Novo Nordic Foundation. The contents of the published materials are solely the responsibility of the Administering Institution of Lillebaelt Hospital and the individual authors identified and do not reflect the views of the research council or the Novo Nordic Foundation. The funders of the study had no role in the study design, data collection, data analysis, data interpretation or writing of the report. The systematic review was completed independently of the administering organization and donors.
REFERENCES
- Tengberg P.T BM, Gromov K, Kallemose T, Troelsen A. Annual Report Danish Fracture Database 2017. [cited 21 September 2021] Available from: https://www.ortopaedi.dk/wp-content/uploads/2019/06/2017.pdf
- Dyer SM, Crotty M, Fairhall N, Magaziner J, Beaupre LA, Cameron ID, et al. A critical review of the long-term disability outcomes following hip fracture. BMC Geriatr 2016; 16: 158. DOI: 10.1186/s12877-016-0332-0
- Abrahamsen C, Nørgaard B. Elderly patients’ perspectives on treatment, care and rehabilitation after hip fracture: a qualitative systematic review. Int J Orthop Trauma Nurs 2021; 41: 100811. DOI: 10.1016/j.ijotn.2020.100811
- Gjertsen J-E, Baste V, Fevang JM, Furnes O, Engesæter LB. Quality of life following hip fractures: results from the Norwegian hip fracture register. BMC Musculoskelet Disord 2016; 17: 265. DOI: 10.1186/s12891-016-1111-y
- Auais MA, Eilayyan O, Mayo NE. Extended exercise rehabilitation after hip fracture improves patients’ physical function: a systematic review and meta-analysis. Phys Ther 2012; 92: 1437–1451. DOI: 10.2522/ptj.20110274
- Fosse RM, Ambugo EA, Moger TA, Hagen TP, Tjerbo T. Does rehabilitation setting influence risk of institutionalization? A register-based study of hip fracture patients in Oslo, Norway. BMC Health Serv Res 2021; 21: 678. DOI: 10.1186/s12913-021-06703-x
- Hulsbæk S, Juhl C, Røpke A, Bandholm T, Kristensen MT. Exercise therapy is effective at improving short- and long-term mobility, ADL and balance in older patients following hip fracture: a systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci 2022; 77: 861–871. DOI: 10.1093/gerona/glab236
- Williamson S, Landeiro F, McConnell T, Fulford-Smith L, Javaid MK, Judge A, et al. Costs of fragility hip fractures globally: a systematic review and meta-regression analysis. Osteoporos Int 2017; 28: 2791–2800. DOI: 10.1007/s00198-017-4153-6
- Drummond M, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. Fourth edn. Oxford: Oxford University Press; 2015.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 2021; 88: 105906. DOI: 10.1016/j.ijsu.2021.105906
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Med 2009; 6: e1000097. DOI: 10.1371/journal.pmed.1000097
- van Mastrigt GA, Hiligsmann M, Arts JJ, Broos PH, Kleijnen J, Evers SM, et al. How to prepare a systematic review of economic evaluations for informing evidence-based healthcare decisions: a five-step approach (part 1/3). Expert Rev Pharmacoecon Outcomes Res 2016: 689–704. DOI: 10.1080/14737167.2016.1246960
- Thielen FW, Van Mastrigt G, Burgers LT, Bramer WM, Majoie H, Evers S, et al. How to prepare a systematic review of economic evaluations for clinical practice guidelines: database selection and search strategy development (part 2/3). Expert Rev Pharmacoecon Outcomes Res 2016; 16: 705–721. DOI: 10.1080/14737167.2016.1246962.
- Wijnen B, Van Mastrigt G, Redekop WK, Majoie H, De Kinderen R, Evers S. How to prepare a systematic review of economic evaluations for informing evidence-based healthcare decisions: data extraction, risk of bias, and transferability (part 3/3). Expert Rev Pharmacoecon Outcomes Res 2016; 16: 723–732. DOI: 10.1080/14737167.2016.1246961
- Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info Libr J 2009; 26: 91–108. DOI: 10.1111/j.1471-1842.2009.00848.x
- Lorbergs AL, MacIntyre NJ. The International Classification of Functioning, Disability and Health (ICF) Core Sets: application to a postmenopausal woman with rheumatoid arthritis and osteoporosis of the spine. Physiother Theory Prac 2013; 29: 547–561. DOI: 10.3109/09593985.2013.773574
- Chrischilles EA, Dasbach EJ, Rubenstein LM, Cook JR, Tabor HK, Black DM, et al. The effect of alendronate on fracture-related healthcare utilization and costs: the fracture intervention trial. Osteoporos Int 2001; 12: 654–660. DOI: 10.1007/s001980170065
- review Wp. Countries with single payer 2021 2021. [cited 4 May 2021] Available from: https://worldpopulationreview.com/country-rankings/countries-with-single-payer
- Watts RD, Li IW. Use of checklists in reviews of health economic evaluations, 2010 to 2018. Value Health 2019; 22: 377–382. DOI: 10.1016/j.jval.2018.10.006
- Welte R, Feenstra T, Jager H, Leidl R. A decision chart for assessing and improving the transferability of economic evaluation results between countries. Pharmacoeconomics 2004; 22: 857–776. DOI: 10.2165/00019053-200422130-00004
- Knies S, Ament AJ, Evers SM, Severens JL. The transferability of economic evaluations: testing the model of Welte. Value Health 2009; 12: 730–738. DOI: 10.1111/j.1524-4733.2009.00525.x
- www.echangerates.org.uk. Exchange rates UK – compare live foreign currency exchange rate & history: UK FX Ltd; [cited 3 October 2021]. Available from: https://www.exchangerates.org.uk/DKK-EUR-spot-exchange-rates-history-2010.html
- Behandlingsrådet. Behandlingsrådets metodevejledning til evaluering af sundhedsteknologi2021; 1.0. [cited 3 October 2021] Available from: https://behandlingsraadet.dk/media/wmuolkyr/metodevejledning.pdf
- Ipsen JA, Pedersen LT, Viberg B, Nørgaard B, Suetta C, Bruun IH. Rehabilitation for life: the effect on physical function of rehabilitation and care in older adults after hip fracture – study protocol for a cluster-randomised stepped-wedge trial. Trials 2022; 23: 375. DOI: 10.1186/s13063-022-06321-w
- Williams N, Dodd S, Hardwick B, Clayton D, Edwards RT, Charles JM, et al. Protocol for a definitive randomised controlled trial and economic evaluation of a community-based rehabilitation programme following hip fracture: fracture in the elderly multidisciplinary rehabilitation-phase III (FEMuR III). BMJ Open 2020; 10: e039791. DOI: 10.1136/bmjopen-2020-039791
- Milte R, Miller MD, Crotty M, Mackintosh S, Thomas S, Cameron ID, et al. Cost-effectiveness of individualized nutrition and exercise therapy for rehabilitation following hip fracture. J Rehabil Med 2016; 48: 378–385. DOI: 10.2340/16501977-2070
- Taraldsen K, Thingstad P, Døhl Ø, Follestad T, Helbostad JL, Lamb SE, et al. Short and long-term clinical effectiveness and cost-effectiveness of a late-phase community-based balance and gait exercise program following hip fracture. The EVA-Hip Randomised Controlled Trial. PLoS One 2019; 14: e0224971. DOI: 10.1371/journal.pone.0224971
- Prestmo A, Hagen G, Sletvold O, Helbostad JL, Thingstad P, Taraldsen K, et al. Comprehensive geriatric care for patients with hip fractures: a prospective, randomised, controlled trial. Lancet 2015; 385: 1623–1633. DOI: 10.1016/S0140-6736(14)62409-0.
- Danske Regioner. Lærings- og Kvalitetetsteams, Hoftenære lårbensbrud 2019. [cited 11 June 2019] Available from: https://kvalitetsteams.dk/laerings-og-kvalitetsteams/lkt-hoftenaere-laarbensbrud
- Conner-Spady B, Marshall D, Bohm E, Dunbar M, Loucks L, Al Khudairy A, et al. Reliability and validity of the EQ-5D-5L compared to the EQ-5D-3L in patients with osteoarthritis referred for hip and knee replacement. Qual Life Res 2015; 24: 1775–1784. DOI: 10.1007/s11136-014-0910-6
- Williamson S, Landeiro F, McConnell T, Fulford-Smith L, Javaid MK, Judge A, et al. Costs of fragility hip fractures globally: a systematic review and meta-regression analysis. Osteoporos Int 2017; 28: 2791–2800. DOI: 10.1007/s00198-017-4153-6.
- Lofgren S, Rehnberg C, Ljunggren G, Brommels M. Coordination pays off: a comparison of two models for organizing hip fracture care, outcomes and costs. Int J Health Plann Manage 2015; 30: 426–438. DOI: 10.1002/hpm.2249
- Devons CA. Comprehensive geriatric assessment: making the most of the aging years. Curr Opin Clin Nutr Metab Care 2002; 5: 19–24. DOI: 10.1097/00075197-200201000-0000
- Cameron ID, Lyle DM, Quine S. Cost effectiveness of accelerated rehabilitation after proximal femoral fracture. J Clin Epidemiol 1994; 47: 1307–1313. DOI: 10.1016/0895-4356(94)90136-8
- Evers SMAA, Hiligsmann M, Adarkwah CC. Risk of bias in trial-based economic evaluations: Identification of sources and bias-reducing strategies. Psychol Health 2015; 30: 52–71. DOI: 10.1080/08870446.2014.953532
- Ariza-Vega P, Ortiz-Pina M, Kristensen MT, Castellote-Caballero Y, Jimenez-Moleon JJ. High perceived caregiver burden for relatives of patients following hip fracture surgery. Disabil Rehabil 2019; 41: 311–318. DOI: 10.1080/09638288.2017.1390612
- van de Ree CLP, Ploegsma K, Kanters TA, Roukema JA, De Jongh MAC, Gosens T. Care-related Quality of Life of informal caregivers of the elderly after a hip fracture. J Patient Rep Outcomes 2017; 2: 23. DOI: 10.1186/s41687-018-0048-3
- Gerkens S, Crott R, Cleemput I, Thissen JP, Closon MC, Horsmans Y, et al. Comparison of three instruments assessing the quality of economic evaluations: a practical exercise on economic evaluations of the surgical treatment of obesity. Int J Technol Assess Health Care 2008; 24: 318–325. DOI: 10.1017/S0266462308080422